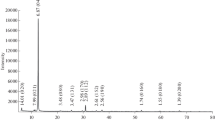
Processes are considered for thermal decomposition of natural (magnesite, brucite) and synthetic (hydromagnesite, hydroxide) magnesium compounds. Thermophysical properties are established for calcination, effect of heating rate, and temperature on phase composition and amount of heat required for calcination. Coefficients determined are the activation energy and pre-exponential factor in an Arrhenius kinetic equation for decomposition of test minerals. It is established that a more convenient form of magnesium-containing product, obtained as a result of chemical enrichment, is hydromagnesite.




Similar content being viewed by others
References
L. M. Aksel’rod, “Development of refractory branch – response to customer requests,” Novye Ogneupory, No. 3, 107 – 122 (2013).
A. N. Smirnov, “General trends in development of refractory material market,” Electronic resource (2014), Access: http://steellab.com.ua/news/2014/01/01.php.
M. A. Shand. The Chemistry and Technology of Magnesia, John Wiley & Sons, Inc. (2006).
D. A. Kramer, Minerals Year Book 2002. Magnesium Compounds, USGS, Reston. Virginia (2002).
Review of the magnesite raw material (magnesite, brucite) market and magnesite powders in the CIS (3rd ed.), INFOMINE Research Group, Infomain, Moscow (2011).
V. N. Zyryanova, “Use of magnesium-containing waste in manufacture of building materials,” Diss. Cand. Techn. Sci., Novosibirsk (1987).
V. A. Perepelitsyn, V. M. Rytvin, V. A. Koroteev, et al., Technogenic Mineral Raw Materials of the Urals [in Russian] RIO UrO RAN, Ekaterinberg (2013).
V. A. Khusnutdinov, “Physicochemical bases of treatment technology for non-traditional magnesia raw material into pure oxide and other magnesium compounds,” Dis. Cand. Techn. Sci., Kazan’ (2000).
V. V. Prokof’eva and Z. V. Bagaushchinov, Magnesia Silicates in the Production of Building Ceramics [in Russian], Zolotoi Orel, St. Petersburg (2005).
L. B. Khoroshavin, V. A. Perepelitsyn, and V. A. Kononov, Magnesia Refractories: Handbook [in Russian, Intermet Inzhiniring, Moscow (2001).
D. A. Kramer, Current Mining of Olivine and Serpentine, U. S. Geological Survey Open-Pile Report, Reston. Virginia (2002).
M. A. Shand, The Chemistry and Technology of Magnesia, John Wiley & Sons, Inc., (2006).
X-Radiography and Physical Metallurgy (Yu. A. Bagardskii, editor) [in Russian], Sci.-Techn. Izd. Lit. Ferrous and Nonferrous Metallurgy, Moscow (1961).
V. P. Ivanov, B. K. Kasatov, T. N. Krasavina, and E. L. Rozinova, Thermal Analysis of Minerals and Rocks [in Russian], Nedra, Leningrad (1974).
L. G. Berg, Introduction to Thermography [in Russian], Izd. Akad Nauk SSSR, Moscow (1961).
V. S. Goroshkov, Thermography of Building Materials [in Russian], Izd. Lit. po-Stroit, Moscow (1968).
B. Roduit, “Computational aspects of kinetic analysis. The ICTAC Kinetics Project — numerical techniques and kinetics of solid state processes,” Thermochim. Acta., 355, 171 – 177 (2000).
C. Liu, et al., “Thermal degradation studies of cyclic olefin copolymers,” Polymer Degradation and Stability, 81, 197 – 205 (2003).
P. Budrugeac and E. Segal, “Application of isoconversional and multivariate non-linear regression methods for evaluation of the degradation mechanism and kinetic parameters of an epoxy resin,” Polymer Degradation and Stability 93(6), 1073 – 1080 (2008). Electronic resource, Access: http://www.sciencedirect. com/science/journal/0413910. Entry data: 09.10.2008.
J. Opfermann, “Kinetic analysis using multivariate nonlinear regression,” J. Thermal Anal. Calorim., 60, 641 – 658 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 10, pp. 28 – 35, October 2015.
Rights and permissions
About this article
Cite this article
Kashcheev, I.D., Zemlyanoi, K.G., Ust’yantsev, V.M. et al. Study of Thermal Decomposition of Natural and Synthetic Magnesium Compounds. Refract Ind Ceram 56, 522–529 (2016). https://doi.org/10.1007/s11148-016-9880-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-016-9880-2