Abstract
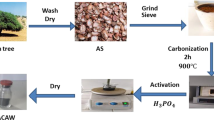
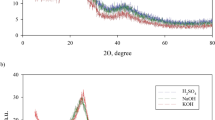
This study aims to valorize agricultural waste of Leucaena leucocephala pods (LP) as a low-cost precursor for synthesizing high-performance activated carbon (LP-AC) for the removal of methylene blue dye (MB). Phosphoric acid H3PO4 was employed as a chemical activator of the LP biomass with a mass ratio of phosphoric acid to the precursor (3/1) before being calcined at 500 °C for 55 min. Box Benken design was investigated to optimize the experimental parameters of initial concentration, adsorbent dose, and pH. Variable optimization indicated that the highest removal efficiency of MB dye, estimated as 99.99%, was noticed at the initial concentration of 300.87 mg L−1, adsorbent dose of 0.049 g, and solution pH of 10.07. Isotherm study revealed that Temkin model shows the best agreement with the experimental data with a correlation coefficient of (R2 = 0.990). The adsorption capacity of MB dye was determined as 584.32 mg g−1. The kinetic study suggested that the pseudo-second-order model is the best-correlated model for data fitting with (R2 > 0.997). The thermodynamic analysis indicated an enthalpy change (ΔH) of − 18.50 kJ/mol, confirming that the adsorption of MB dye onto LP-AC material is an exothermic process. SEM characterization of the surface showed that the LP-AC exhibits a heterogeneous structure. The BET analysis revealed a remarkable surface area of 1367.30 m2 g−1 for the produced carbon, including a blend of mesoporous and microporous structures. Furthermore, complementary analyses including EDS, TGA, and FTIR confirmed the presence of crucial properties, underscoring its potential effectiveness as an adsorbent for removing MB dye.







Similar content being viewed by others
References
Gohr MS, Abd-Elhamid AI, El-Shanshory AA, Soliman HM (2022) Adsorption of cationic dyes onto chemically modified activated carbon: kinetics and thermodynamic study. J Mol Liq 346:118227. https://doi.org/10.1016/j.molliq.2021.118227
Bhatnagar A, Hogland W, Marques M, Sillanpää M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511. https://doi.org/10.1016/j.cej.2012.12.038
Zhao W-d, Chen L-P, Jiao Y (2023) Preparation of activated carbon from sunflower straw through H3PO4 activation and its application for acid fuchsin dye adsorption. Water Sci Eng 16:192–202. https://doi.org/10.1016/j.wse.2023.02.002
Boudghene Stambouli H, Guenfoud F, Benomara A et al (2021) Synthesis of FeWO4 heterogeneous composite by the sol–gel process: enhanced photocatalytic activity on malachite green. Reac Kinet Mech Cat 133:563–578. https://doi.org/10.1007/s11144-021-01994-x
Yueqin Yu, Zhao C, Liu X, Sui M, Meng Y (2017) Selective flocculation of pollutants in wastewater using pH responsive HM-alginate/chitosan complexes. J Environ Chem Eng 5:5406–5410. https://doi.org/10.1016/j.jece.2017.10.025
Liu H, Zhang J, Lu M et al (2020) Biosynthesis based membrane filtration coupled with iron nanoparticles reduction process in removal of dyes. Chem Eng J 387:124202. https://doi.org/10.1016/j.cej.2020.124202
Ikram M, Zahoor M, Batiha GE-S (2021) Biodegradation and decolorization of textile dyes by bacterial strains: a biological approach for wastewater treatment. Z Phys Chem 235:1381–1393. https://doi.org/10.1515/zpch-2020-1708
Popovici DR, Neagu M, Dusescu-Vasile CM et al (2021) Adsorption of p-nitrophenol onto activated carbon prepared from fir sawdust: isotherm studies and error analysis. Reac Kinet Mech Cat 133:483–500. https://doi.org/10.1007/s11144-021-01997-8
Li X, Tang S, Yuan D et al (2019) Improved degradation of anthraquinone dye by electrochemical activation of PDS. Ecotoxicol Environ Saf 177:77–85. https://doi.org/10.1016/j.ecoenv.2019.04.015
Mohamed FF, Allah PM, Mehdi AP, Baseem M (2011) Photoremoval of malachite green (MG) using advanced oxidation process. Res J Chem Environ 15:65–70
Surip SN, Abdulhameed AS, Garba ZN et al (2020) H2SO4-treated Malaysian low rank coal for methylene blue dye decolourization and cod reduction: optimization of adsorption and mechanism study. Surf Interfaces 21:100641. https://doi.org/10.1016/j.surfin.2020.100641
Jawad AH, Mastuli MS, Mallah S, Mastuli S (2019) Adsorption behavior of methylene blue on acid-treated rubber (Hevea brasiliensis) leaf. Desalin Water Treat 124:297–307. https://doi.org/10.5004/dwt.2018
**e J, Lin R, Liang Z et al (2021) Effect of cations on the enhanced adsorption of cationic dye in Fe3O4-loaded biochar and mechanism. J Environ Chem Eng 9:105744. https://doi.org/10.1016/j.jece.2021.105744
Igwegbe CA, Onukwuli OD, Ighalo JO, Okoye PU (2020) Adsorption of cationic dyes on Dacryodes edulis seeds activated carbon modified using phosphoric acid and sodium chloride. Environ Process 7:1151–1171. https://doi.org/10.1007/s40710-020-00467-y
Luo Y, Li D, Chen Y et al (2019) The performance of phosphoric acid in the preparation of activated carbon-containing phosphorus species from rice husk residue. J Mater Sci 54:5008–5021. https://doi.org/10.1007/s10853-018-03220-x
Pang X, Sellaoui L, Franco D et al (2020) Preparation and characterization of a novel mountain soursop seeds powder adsorbent and its application for the removal of crystal violet and methylene blue from aqueous solutions. Chem Eng J 391:123617. https://doi.org/10.1016/j.cej.2019.123617
Pathania D, Sharma S, Singh P (2017) Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab J Chem 10:S1445–S1451. https://doi.org/10.1016/j.arabjc.2013.04.021
Al-Qaim FF, Al-Saedi HFS, Mussa ZH et al (2024) Application of the response surface approach to the adsorption of methylene blue from water using acid-modified grape leaves. Reac Kinet Mech Cat 137:399–422. https://doi.org/10.1007/s11144-023-02542-5
Bageel A, Honda MDH, Carrillo JT, Borthakur D (2020) Giant leucaena (Leucaena leucocephala subsp. glabrata): a versatile tree-legume for sustainable agroforestry. Agroforest Syst 94:251–268. https://doi.org/10.1007/s10457-019-00392-6
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. J Taibah Univ Sci 14:305–313. https://doi.org/10.1080/16583655.2020.1736767
Ahmed MJ (2016) Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption. J Environ Chem Eng 4:89–99. https://doi.org/10.1016/j.jece.2015.10.027
Yahya MA, Al-Qodah Z, Ngah CZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235. https://doi.org/10.1016/j.rser.2015.02.051
Martínez de Yuso A, Rubio B, Izquierdo MT (2014) Influence of activation atmosphere used in the chemical activation of almond shell on the characteristics and adsorption performance of activated carbons. Fuel Process Technol 119:74–80. https://doi.org/10.1016/j.fuproc.2013.10.024
Yusuff AS (2019) Adsorption of hexavalent chromium from aqueous solution by Leucaena leucocephala seed pod activated carbon: equilibrium, kinetic and thermodynamic studies. Arab J Basic Appl Sci 26:89–102. https://doi.org/10.1080/25765299.2019.1567656
Patidar K, Vashishtha M (2020) Optimization of process variables to prepare mesoporous activated carbon from mustard straw for dye adsorption using response surface methodology. Water Air Soil Pollut 231:526. https://doi.org/10.1007/s11270-020-04893-4
Francoeur M, Ferino-Pérez A, Yacou C et al (2021) Activated carbon synthetized from Sargassum (sp) for adsorption of caffeine: understanding the adsorption mechanism using molecular modeling. J Environ Chem Eng 9:104795. https://doi.org/10.1016/j.jece.2020.104795
Jawad AH, Mohd Firdaus Hum NN, Abdulhameed AS, Mohd Ishak MA (2022) Mesoporous activated carbon from grass waste via H3PO4-activation for methylene blue dye removal: modelling, optimisation, and mechanism study. Int J Environ Anal Chem 102:6061–6077. https://doi.org/10.1080/03067319.2020.1807529
Oginni O, Singh K, Oporto G et al (2019) Effect of one-step and two-step H3PO4 activation on activated carbon characteristics. Biores Technol Rep 8:100307. https://doi.org/10.1016/j.biteb.2019.100307
Belhamdi B, Merzougui Z, Laksaci H, Trari M (2019) The removal and adsorption mechanisms of free amino acid l-tryptophan from aqueous solution by biomass-based activated carbon by H3PO4 activation: regeneration study. Phys Chem Earth A/B/C 114:102791. https://doi.org/10.1016/j.pce.2019.07.004
Li X, Han D, Zhang M et al (2019) Removal of toxic dyes from aqueous solution using new activated carbon materials developed from oil sludge waste. Colloids Surf A 578:123505. https://doi.org/10.1016/j.colsurfa.2019.05.066
Solomon D, Kiflie Z, Van Hulle S (2020) Using Box–Behnken experimental design to optimize the degradation of Basic Blue 41 dye by Fenton reaction. Int J Ind Chem 11:43–53. https://doi.org/10.1007/s40090-020-00201-5
Sadaf S, Bhatti HN (2016) Response surface methodology approach for optimization of adsorption process for the removal of Indosol Yellow BG dye from aqueous solution by agricultural waste. Desalin Water Treat 57:11773–11781. https://doi.org/10.1080/19443994.2015.1048308
Okolo BI, Oke EO, Agu CM et al (2020) Adsorption of lead(II) from aqueous solution using Africa elemi seed, mucuna shell and oyster shell as adsorbents and optimization using Box–Behnken design. Appl Water Sci 10:1–23. https://doi.org/10.1007/s13201-020-01242-y
Ahmadi S, Mohammadi L, Rahdar A et al (2020) Acid dye removal from aqueous solution by using neodymium(III) oxide nanoadsorbents. Nanomaterials 10:556. https://doi.org/10.3390/nano10030556
Bedada D, Angassa K, Tiruneh A et al (2020) Chromium removal from tannery wastewater through activated carbon produced from Parthenium hysterophorus weed. Energ Ecol Environ 5:184–195. https://doi.org/10.1007/s40974-020-00160-8
Okpara OG, Ogbeide OM, Ike OC et al (2021) Optimum isotherm by linear and nonlinear regression methods for lead(II) ions adsorption from aqueous solutions using synthesized coconut shell–activated carbon (SCSAC). Toxin Reviews 40:901–914. https://doi.org/10.1080/15569543.2020.1802596
Hadi M, Samarghandi MR, McKay G (2010) Equilibrium two-parameter isotherms of acid dyes sorption by activated carbons: study of residual errors. Chem Eng J 160:408–416. https://doi.org/10.1016/j.cej.2010.03.016
Manna S, Roy D, Saha P et al (2017) Rapid methylene blue adsorption using modified lignocellulosic materials. Process Saf Environ Prot 107:346–356. https://doi.org/10.1016/j.psep.2017.03.008
Mbarki F, Selmi T, Kesraoui A, Seffen M (2022) Low-cost activated carbon preparation from corn stigmata fibers chemically activated using H3PO4, ZnCl2 and KOH: study of methylene blue adsorption, stochastic isotherm and fractal kinetic. Ind Crops Prod 178:114546. https://doi.org/10.1016/j.indcrop.2022.114546
Raji Y, Nadi A, Mechnou I et al (2023) High adsorption capacities of crystal violet dye by low-cost activated carbon prepared from Moroccan Moringa oleifera wastes: characterization, adsorption and mechanism study. Diam Relat Mater 135:109834. https://doi.org/10.1016/j.diamond.2023.109834
Fennouh R, Benturki O, Mokhati A et al (2023) Preparation and characterization of highly mesoporous activated carbon from Ziziphus Spina-Christi for tartrazine adsorption from a simulated effluent. Biomass Conv Bioref. https://doi.org/10.1007/s13399-023-04296-5
Yilmaz P, Gunduz D, Ozbek B (2021) Utilization of low-cost bio-waste adsorbent for methylene blue dye removal from aqueous solutions and optimization of process variables by response surface methodology approach. Desal Water Treat 224:367–388. https://doi.org/10.5004/dwt.2021.27206
Afroze S, Sen TK, Ang M, Nishioka H (2016) Adsorption of methylene blue dye from aqueous solution by novel biomass Eucalyptus sheathiana bark: equilibrium, kinetics, thermodynamics and mechanism. Desalin Water Treat 57:5858–5878. https://doi.org/10.1080/19443994.2015.1004115
Liu H, Zhang J, Bao N et al (2012) Textural properties and surface chemistry of lotus stalk-derived activated carbons prepared using different phosphorus oxyacids: adsorption of trimethoprim. J Hazard Mater 235:367–375. https://doi.org/10.1016/j.jhazmat.2012.08.015
Yorgun S, Yıldız D (2015) Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J Taiwan Inst Chem Eng 53:122–131. https://doi.org/10.1016/j.jtice.2015.02.032
Nabih MH, El Hajam M, Boulika H et al (2023) Preparation and characterization of activated carbons from cardoon “Cynara Cardunculus” waste: application to the adsorption of synthetic organic dyes. Mater Today 72:3369–3379. https://doi.org/10.1016/j.matpr.2022.07.414
Nasrullah A, Saad B, Bhat AH et al (2019) Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: characterization and application for methylene blue removal. J Clean Prod 211:1190–1200. https://doi.org/10.1016/j.jclepro.2018.11.094
Netto MS, Georgin J, Franco DSP et al (2022) Effective adsorptive removal of atrazine herbicide in river waters by a novel hydrochar derived from Prunus serrulata bark. Environ Sci Pollut Res 29:3672–3685. https://doi.org/10.1007/s11356-021-15366-4
Koyuncu F, Güzel F, Sayğılı H (2018) Role of optimization parameters in the production of nanoporous carbon from mandarin shells by microwave-assisted chemical activation and utilization as dye adsorbent. Adv Powder Technol 29:2108–2118. https://doi.org/10.1016/j.apt.2018.05.019
Chimi T, Hannah BU, Lincold NM et al (2023) Preparation, characterization and application of H3PO4-activated carbon from Pentaclethra macrophylla pods for the removal of Cr(VI) in aqueous medium. J Iran Chem Soc 20:399–413. https://doi.org/10.1007/s13738-022-02675-9
Attallah OA, Mamdouh W (2021) Development and optimization of pectin/chitosan magnetic sponge for efficient cationic dyes removal using Box–Behnken design. Int J Environ Sci Technol 18:131–140. https://doi.org/10.1007/s13762-020-02828-4
Afshin S, Rashtbari Y, Vosough M et al (2021) Application of Box–Behnken design for optimizing parameters of hexavalent chromium removal from aqueous solutions using Fe3O4 loaded on activated carbon prepared from alga: kinetics and equilibrium study. J Water Process Eng 42:102113. https://doi.org/10.1016/j.jwpe.2021.102113
Sahu S, Pahi S, Tripathy S et al (2020) Adsorption of methylene blue on chemically modified lychee seed biochar: dynamic, equilibrium, and thermodynamic study. J Mol Liq 315:113743. https://doi.org/10.1016/j.molliq.2020.113743
Sen K, Mondal NK, Chattoraj S, Datta JK (2017) Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology. Environ Earth Sci 76:1–15. https://doi.org/10.1007/s12665-016-6333-7
Ahmad NH, Mohamed MA, Yusoff SFM (2020) Improved adsorption performance of rubber-based hydrogel: optimisation through response surface methodology, isotherm, and kinetic studies. J Sol–Gel Sci Technol 94:322–334. https://doi.org/10.1007/s10971-020-05254-7
Takele T, Angassa K, Abewaa M et al (2023) Adsorption of methylene blue from textile industrial wastewater using activated carbon developed from H3PO4-activated khat stem waste. Biomass Conv Bioref. https://doi.org/10.1007/s13399-023-05245-y
Martins AC, Pezoti O, Cazetta AL et al (2015) Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: kinetic and equilibrium studies. Chem Eng J 260:291–299. https://doi.org/10.1016/j.cej.2014.09.017
Üner O, Geçgel Ü, Bayrak Y (2016) Adsorption of methylene blue by an efficient activated carbon prepared from Citrullus lanatus Rind: kinetic, isotherm, thermodynamic, and mechanism analysis. Water Air Soil Pollut 227:247. https://doi.org/10.1007/s11270-016-2949-1
Attia AA, Girgis BS, Khedr SA (2003) Capacity of activated carbon derived from pistachio shells by H3PO4 in the removal of dyes and phenolics. J Chem Technol Biotechnol 78:611–619. https://doi.org/10.1002/jctb.743
Jawad AH, Bardhan M, Islam MA et al (2020) Insights into the modeling, characterization and adsorption performance of mesoporous activated carbon from corn cob residue via microwave-assisted H3PO4 activation. Surf Interfaces 21:100688. https://doi.org/10.1016/j.surfin.2020.100688
Ibrahim M, Souleiman M, Salloum A (2023) Methylene blue dye adsorption onto activated carbon developed from Calicotome villosa via H3PO4 activation. Biomass Conv Bioref 13:12763–12776. https://doi.org/10.1007/s13399-021-02027-2
Belaissa Y, Saib F, Trari M (2022) Removal of amoxicillin in aqueous solutions by a chemical activated carbons derived from Jujube nuts: adsorption behaviors, kinetic and thermodynamic studies. Reac Kinet Mech Cat 135:1011–1030. https://doi.org/10.1007/s11144-022-02159-0
Mozhiarasi V, Natarajan TS (2022) Bael fruit shell-derived activated carbon adsorbent: effect of surface charge of activated carbon and type of pollutants for improved adsorption capacity. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-03211-8
Baytar O, Ceyhan AA, Şahin Ö (2020) Production of activated carbon from Elaeagnus angustifolia seeds using H3PO4 activator and methylene blue and malachite green adsorption. Int J Phytoremediat. https://doi.org/10.1080/15226514.2020.1849015
Daniel LS, Rahman A, Hamushembe MN et al (2023) The production of activated carbon from Acacia erioloba seedpods via phosphoric acid activation method for the removal of methylene blue from water. Biores Technol Rep 23:101568. https://doi.org/10.1016/j.biteb.2023.101568
Li M, Mu J, Liu Y et al (2023) Removal of phenol by lignin-based activated carbon as an efficient adsorbent for adsorption of phenolic wastewater. Res Chem Intermed 49:2209–2232. https://doi.org/10.1007/s11164-023-04958-z
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laouar, N.E.H., Boukerroui, A., Meziti, C. et al. Adsorption study of methylene blue dye removal with activated carbon derived from Leucaena leucocephala wastes prepared via H3PO4 activation. Reac Kinet Mech Cat (2024). https://doi.org/10.1007/s11144-024-02677-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11144-024-02677-z