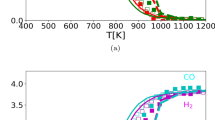
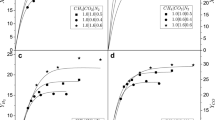
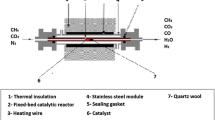
Abstract
The dry reforming of methane (DRM) was conducted using a Ni-SiO2 catalysts. It was evaluated the catalyst’s stability within the temperature range of 973–1033 K under low spatial time conditions (kinetic regime) in the reactor. The catalyst deactivated more rapidly at high temperatures, contrary to the prediction based on chemical equilibrium calculations. To understand this behavior, it was investigated the impact of reactor operating conditions by simulating the reactor using a pseudo-homogeneous model and comparing the results with the chemical equilibrium prediction. The simulations revealed that, at high spatial times (W/FCH4), the reactions considered for the DRM process closely approach equilibrium. In contrast, at low spatial times, the reactive system deviates from chemical equilibrium, with the water gas shift and disproportionation of CO being the most favored. This results in increased coke production, which leads to faster catalyst deactivation. The effect is attributed to the kinetic inhibition of CO2 adsorption, hindering the activation of this molecule at high spatial time. The spatial time in the reactor, whether high or low, strongly depends on the intrinsic catalytic activity of the catalyst. Thus, when studying catalytic solids, the operating conditions of the reactor should be taken into account to avoid erroneous interpretations of the experimental data when evaluating their performance (for example, catalyst selectivity or resistance against deactivation by coke).








Similar content being viewed by others
Abbreviations
- \({K}_{i}(T)\) :
-
Theoretical equilibrium coefficient in function of temperature
- \({T}_{Ref}\) :
-
Temperature for the reference (298 K)
- \(\Delta {G}_{Ref}^{o}\) :
-
Free Gibbs energy in standard conditions
- \(\Delta {H}_{r}\) :
-
Enthalpy of the chemical reaction
- \({r}_{i}\) :
-
Reaction rate for the chemical reaction i
- \({k}_{i}\) :
-
Kinetic coefficient of reaction i
- \({F}_{i}\) :
-
Molar flow of the compound i
- \({P}_{T}\) :
-
Total pressure of the reaction system
- \({K}_{P}\) :
-
Equilibrium coefficient of the reaction rate
- \({K}_{i,j}\) :
-
Adsorption coefficient for the compound i in the reaction j
- \({F}_{T}\) :
-
Total molar flow
- \(L\) :
-
Reactor Length
- \({D}_{R}\) :
-
Diameter of the tubular reactor
- \({\rho }_{cat}\) :
-
Density of the catalyst bed
- \(\frac{d{F}_{i}}{dL}\) :
-
Differential change of the molar flow of the i compound with reactor length
- \(\frac{dT}{dL}\) :
-
Differential change of the temperature with reactor length
- \({U}_{w}\) :
-
Global heat transfer coefficient
- \({T}_{w}\) :
-
Temperature of the reactor wall
- \({p}_{j}^{n}\) :
-
Partial pressure of component j as product
- \({a}_{j}^{n}\) :
-
Partial pressure of component j as reactant
- \(n\) :
-
Stoichiometric coefficient of component j
References
Sandoval-Diaz LE, Schlögl R, Lunkenbein T (2022) Quo Vadis dry reforming of methane? A review on its chemical, environmental, and industrial prospects. Catalysts 12:465. https://doi.org/10.3390/catal12050465
Wittich K, Krämer M, Bottke N, Schunk SA (2020) Catalytic dry reforming of methane: insights from model systems. ChemCatChem 12:2130–2147. https://doi.org/10.1002/cctc.201902142
Li M, Sun Z, Hu YH (2022) Thermo-photo coupled catalytic CO2 reforming of methane: a review. Chem Eng J 428:131222. https://doi.org/10.1016/j.cej.2021.131222
Vakili R, Gholami R, Stere CE et al (2020) Plasma-assisted catalytic dry reforming of methane (DRM) over metal-organic frameworks (MOFs)-based catalysts. Appl Catal B Environ 260:118195. https://doi.org/10.1016/j.apcatb.2019.118195
Li M, Sun Z, Hu YH (2021) Catalysts for CO2 reforming of CH4: a review. J Mater Chem A 9:12495–12520. https://doi.org/10.1039/D1TA00440A
Alenazey FSh (2014) Utilizing carbon dioxide as a regenerative agent in methane dry reforming to improve hydrogen production and catalyst activity and longevity. Int J Hydrog Energy 39:18632–18641. https://doi.org/10.1016/j.ijhydene.2014.02.148
Owgi AHK, Jalil AA, Hussain I et al (2021) Catalytic systems for enhanced carbon dioxide reforming of methane: a review. Environ Chem Lett 19:2157–2183. https://doi.org/10.1007/s10311-020-01164-w
Lavoie JM (2014) Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front Chem 2:81. https://doi.org/10.3389/fchem.2014.00081
Iyer MV, Norcio LP, Kugler EL, Dadyburjor DB (2003) Kinetic modeling for methane reforming with carbon dioxide over a mixed-metal carbide catalyst. Ind Eng Chem Res 42:2712–2721. https://doi.org/10.1021/ie020677q
Bradford MCJ, Vannice MA (1999) CO2 reforming of CH4. Catal Rev 41:1–42. https://doi.org/10.1081/CR-100101948
Bartholomew CH (2001) Mechanisms of catalyst deactivation. Appl Catal Gen 212:17–60. https://doi.org/10.1016/S0926-860X(00)00843-7
Rostrup-Nielsen JR (1997) Industrial relevance of coking. Catal Today 37:225–232. https://doi.org/10.1016/S0920-5861(97)00016-3
Zambrano D, Soler J, Herguido J, Menéndez M (2019) Kinetic study of dry reforming of methane over Ni–Ce/Al2O3 catalyst with deactivation. Top Catal 62:456–466. https://doi.org/10.1007/s11244-019-01157-2
Ginsburg JM, Piña J, El Solh T, de Lasa HI (2005) Coke formation over a nickel catalyst under methane dry reforming conditions: thermodynamic and kinetic models. Ind Eng Chem Res 44:4846–4854. https://doi.org/10.1021/ie0496333
Benguerba Y, Dehimi L, Virginie M et al (2015) Modelling of methane dry reforming over Ni/Al2O3 catalyst in a fixed-bed catalytic reactor. React Kinet Mech Catal 114:109–119. https://doi.org/10.1007/s11144-014-0772-5
Delgado KH, Maier L, Tischer S et al (2015) Surface reaction kinetics of steam- and CO2-reforming as well as oxidation of methane over nickel-based catalysts. Catalysts 5:871–904. https://doi.org/10.3390/catal5020871
Rakhi GV, Mauss F (2023) Insights into dry reforming of methane over nickel catalyst using a thermodynamic model. React Kinet Mech Catal 136:1197–1210. https://doi.org/10.1007/s11144-023-02426-8
Scilab, version 6.1.0. https://www.scilab.org. Accessed 28 Apr 2024
DWSIM—The Open Source Chemical Process Simulator (2024) Chemical process simulation for everyone: DWSIM for desktop is free and open-source. https://dwsim.org/. Accessed 28 Apr 2024
Cherbański R, Franczyk E, Lewak M et al (2021) Modelling of methane dry reforming over Ni/CaO–Al2O3 catalyst. Chem Process Eng 42(3):235–252
Raybaut P (2009) Spyder-documentation. https://www.spyder-ide.org/. Accessed 28 Apr 2024
de la Cruz-Flores VG, Martinez-Hernandez A, Gracia-Pinilla MA (2020) Deactivation of Ni-SiO2 catalysts that are synthetized via a modified direct synthesis method during the dry reforming of methane. Appl Catal Gen 594:117455. https://doi.org/10.1016/j.apcata.2020.117455
Moulijn JA, van Diepen AE, Kapteijn F (2001) Catalyst deactivation: is it predictable?: what to do? Appl Catal Gen 212:3–16. https://doi.org/10.1016/S0926-860X(00)00842-5
Froment GF (2001) Modeling of catalyst deactivation. Appl Catal Gen 212:117–128. https://doi.org/10.1016/S0926-860X(00)00850-4
Kahle LCS, Roussière T, Maier L et al (2013) Methane dry reforming at high temperature and elevated pressure: impact of gas-phase reactions. Ind Eng Chem Res 52:11920–11930. https://doi.org/10.1021/ie401048w
Abdel Karim Aramouni N, Zeaiter J, Kwapinski W, Ahmad MN (2017) Thermodynamic analysis of methane dry reforming: effect of the catalyst particle size on carbon formation. Energy Convers Manag 150:614–622. https://doi.org/10.1016/j.enconman.2017.08.056
Vogt C, Kranenborg J, Monai M, Weckhuysen BM (2020) Structure sensitivity in steam and dry methane reforming over nickel: activity and carbon formation. ACS Catal 10:1428–1438. https://doi.org/10.1021/acscatal.9b04193
Barroso Quiroga MM, Castro Luna AE (2007) Kinetic analysis of rate data for dry reforming of methane. Ind Eng Chem Res 46:5265–5270. https://doi.org/10.1021/ie061645w
Nikoo MK, Amin NAS (2011) Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process Technol 92:678–691. https://doi.org/10.1016/j.fuproc.2010.11.027
Calgaro CO, Rocha AL, Perez-Lopez OW (2020) Deactivation control in CO2 reforming of methane over Ni–Mg–Al catalyst. React Kinet Mech Catal 130:159–178. https://doi.org/10.1007/s11144-020-01770-3
Al-Otaibi F, **ao H, Berrouk AS, Polychronopoulou K (2023) Numerical study of dry reforming of methane in packed and fluidized beds: effects of key operating parameters. ChemEng 7:57. https://doi.org/10.3390/chemengineering7030057
Acknowledgements
The author gratefully acknowledges the contribution of Eric Flores-Elizondo and Victor G. de la Cruz-Flores, who obtained part of the experimental results during their studies to obtain a master’s degree.
Funding
This work was supported by grants from CONACYT (Project No. 156064) and UANL (PAICYT No. IT550-10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author has no competing interests to declare that are relevant to the content of this article.
Content of the publication
During the preparation of this work, the author used ARIA and Copilot (Opera and Microsoft Edge Browsers) in order to improve language and readability, and the author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martinez-Hernandez, A. Dry reforming of methane with a Ni-based catalyst: a kinetic and thermodynamic analysis. Reac Kinet Mech Cat (2024). https://doi.org/10.1007/s11144-024-02658-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11144-024-02658-2