Abstract
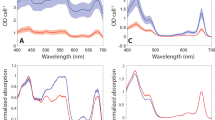
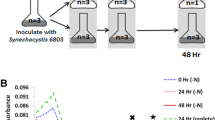
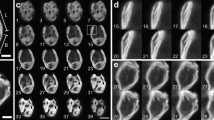
Photosynthetic organisms rely on antenna systems to harvest and deliver energy from light to reaction centers. In fluctuating photic environments, regulation of light harvesting is critical for a photosynthetic organism’s survival. Here, we describe the use of a suite of phycobilisome mutants to probe the consequences of antenna truncation in the cyanobacterium Synechocystis sp. PCC 6803. Studies using transmission electron microscopy (TEM), hyperspectral confocal fluorescence microscopy (HCFM), small-angle neutron scattering (SANS), and an optimized photobioreactor system have unraveled the adaptive strategies that cells employ to compensate for antenna reduction. As the phycobilisome antenna size decreased, changes in thylakoid morphology were more severe and physical segregation of the two photosystems increased. Repeating distances between thylakoid membranes measured by SANS were correlated with TEM data, and corresponded to the degree of phycobilisome truncation. Thylakoid membranes were found to have a high degree of structural flexibility, and changes in the membrane system upon illumination were rapid and reversible. Phycobilisome truncation in Synechocystis 6803 reduced the growth rate and lowered biomass accumulation. Together, these results lend a dynamic perspective to the intracellular membrane organization in cyanobacteria cells and suggest an adaptive mechanism that allows cells to adjust to altered light absorption capabilities, while highlighting the cell-wide implications of antenna truncation.




Similar content being viewed by others
References
Adir N (2005) Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth Res 85:15–32
Ajlani G, Vernotte C (1998) Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol Biol 37(3):577–580
Ajlani G, Vernotte C et al (1995) Phycobilisome core mutants of Synechocystis PCC 6803. Biochem Biophys Acta 1231:189–196
Arteni AA, Ajlani G et al (2009) Structural organisation of phycobilisomes from Synechocystis sp. strain PCC6803 and their interaction with the membrane. Biochimi Biophys Acta Bioenergetics 1787(4):272–279
Blankenship RE (2002) Molecular mechanisms of photosynthesis. Blackwell Science, Oxford
Collins AM, Liberton M et al (2012) Photosynthetic pigment localization and thylakoid membrane morphology are altered in Synechocystis 6803 phycobilisome mutants. Plant Physiol 158(4):1600–1609
Elmorjani K, Thomas J-C et al (1986) Phycobilisomes of wild type and pigment mutants of the cyanobacterium Synechocystis PCC 6803. Arch Microbiol 146:186–191
Liberton M, Page LE et al (2013) Organization and flexibility of cyanobacterial thylakoid membranes examined by neutron scattering. J Biol Chem 288(5):3632–3640
MacColl R (1998) Cyanobacterial phycobilisomes. J Struct Biol 124(2–3):311–334
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177(4):272–280
Mullineaux CW (1999) The thylakoid membranes of cyanobacteria: structure, dynamics and function. Aust J Plant Physiol 26:671–677
Mullineaux C (2008) Phycobilisome-reaction centre interaction in cyanobacteria. Photosynth Res 95(2):175–182
Nagy G, Posselt D et al (2011) Reversible membrane reorganizations during photosynthesis in vivo: revealed by small-angle neutron scattering. Biochem J 436:225–230
Nakajima Y, Ueda R (1997) Improvements of photosynthesis in dense microalgal suspension by reduction of light harvesting pigments. J Appl Phycol 9:503–510
Nedbal L, Trtílek M et al (2008) A photobioreactor system for precision cultivation of photoautotrophic microorganisms and for high-content analysis of suspension dynamics. Biotechnol Bioeng 100(5):902–910
Olive J (1997) Ultrastructure and light adaptation of phycobilisome mutants of Synechocystis PCC 6803. Biochimi Biophys Acta Bioenergetics 1319(2–3):275–282
Ort DR, Melis A (2011) Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol 155(1):79–85
Page LE, Liberton M et al (2012) Reduction of photoautotrophic productivity in the cyanobacterium Synechocystis sp. strain PCC 6803 by phycobilisome antenna truncation. Appl Environ Microbiol 78(17):6349–6351
Perrine Z, Negi S et al (2012) Optimization of photosynthetic light energy utilization by microalgae. Algal Res 1:134–142
Schoonover JR, Marx R et al (2003) Multivariate curve resolution in the analysis of vibrational spectroscopy data files. Appl Spectrosc 57(5):154A–170A
Sherman DM, Troyan TA et al (1994) Localization of membrane proteins in the cyanobacterium Synechococcus sp. PCC7942 (radial asymmetry in the photosynthetic complexes. Plant Physiol 106:251–262
Sidler WA (1994) Phycobilisome and phycobiliprotein structures. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer Academic Publishers, The Netherlands, pp 139–216
Stadnichuk I, Lukashev E et al (2009) Increase in the rate of photosynthetic linear electron transport in cyanobacteruim Synechocystis sp. PCC 6803 lacking phycobilisomes. Russ J Plant Physiol 56(4):439–444
Tanaka A, Ito H et al (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci 95(21):12719–12723
Tang KH, Blankenship RE (2012) Neutron and light scattering studies of light-harvesting photosynthetic antenna complexes. Photosynth Res 111(1–2):205–217
Tauler R (1995) Multivariate curve resolution applied to second order data. Chemom Intell Lab Syst 30(1):133–146
Ughy B, Ajlani G (2004) Phycobilisome rod mutants in Synechocystis sp. strain PCC6803. Microbiology 150(12):4147
van de Meene AM, Hohmann-Marriott MF et al (2006) The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch Microbiol 184(5):259–270
Vermaas WF, Timlin JA et al (2008) In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells. Proc Natl Acad Sci USA 105(10):4050–4055
Acknowledgments
The authors thank Ghada Ajlani for the kind gift of the phycobilisome mutant strains described in this study, Michael Sinclair for the maintenance and use of the hyperspectral confocal fluorescence microscope, and Howland Jones for the development of the multivariate analysis software. The authors also thank Howard Berg of the Donald Danforth Plant Science Center’s Integrated Microscopy Facility for TEM assistance. This material is based upon work supported as part of the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC 0001035.
The Bio-SANS instrument is a resource of the Center for Structural Molecular Biology at Oak Ridge National Laboratory that is supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research Project ERKP291. Bio-SANS is located at the Oak Ridge National Laboratory’s High Flux Isotope Reactor. The neutron source is sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy.
Sandia National Laboratories is a multi-program laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation, for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liberton, M., Collins, A.M., Page, L.E. et al. Probing the consequences of antenna modification in cyanobacteria. Photosynth Res 118, 17–24 (2013). https://doi.org/10.1007/s11120-013-9940-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9940-0