Abstract
Aims
In view of the projected increase in global air temperature and CO2 concentration, the effects of climatic changes on biomass production, CO2 fluxes and arbuscular mycorrhizal fungi (AMF) colonization in newly established grassland communities were investigated. We hypothesized that above- and below-ground biomass, gross primary productivity (GPP), AMF root colonization and nutrient acquisition would increase in response to the future climate conditions. Furthermore, we expected that increased below-ground C allocation would enhance soil respiration (Rsoil).
Methods
Grassland communities were grown either at ambient temperatures with 375 ppm CO2 (Amb) or at ambient temperatures +3°C with 620 ppm CO2 (T+CO2).
Results
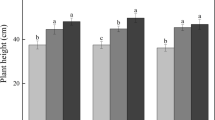
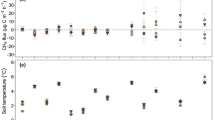
Total biomass production and GPP were stimulated under T+CO2. Above-ground biomass was increased under T+CO2 while belowground biomass was similar under both climates. The significant increase in root colonization intensity under T+CO2, and therefore the better contact between roots and AMF, probably determined the higher above-ground P and N content. Rsoil was not significantly affected by the future climate conditions, only showing a tendency to increase under future climate at the end of the season.
Conclusions
Newly established grasslands benefited from the exposure to elevated CO2 and temperature in terms of total biomass production; higher root AMF colonization may partly provide the nutrients required to sustain this growth response.





Similar content being viewed by others
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Ainsworth EA, Davey PA, Hymus GJ, Osborne CP, Rogers A, Blum H, Nösberger J, Long SP (2003) Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for ten years at two nitrogen fertilization levels under Free Air CO2 Enrichment (FACE). Plant Cell Environ 26:705–714
Ames RN, Reid CPP, Porter LK, Cambardella C (1983) Hyphal uptake and transport of nitrogen from two 15N-labeled sources by Glomus mosseae, a vesicular arbuscular mycorrhizal fungus. New Phytol 95:381–396
Ammann C, Flechard CR, Leifeld J, Neftel A, Fuhrer J (2007) The carbon budget of newly established temperate grassland depends on management intensity. Agric Ecosyst Environ 121:5–20
Arnone JA, Zaller JG, Spehn EM, Niklaus PA, Wells CE, Körner C (2000) Dynamics of root systems in native grasslands: effects of elevated CO2. New Phytol 147:73–85
Atkin OK, Scheurwater I, Pons TL (2006) High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Global Change Biol 12:500–515
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Chen X, Tu C, Burton MG, Watson DM, Burkey KO, Hu S (2007) Plant nitrogen acquisition and interactions under elevated carbon dioxide: impact of endophytes and mycorrhizae. Global Change Biol 13:1238–1249
Comstedt D, Boström B, Marshall JD, Holm A, Slaney M, Linder S, Ekblad A (2006) Effects of elevated [CO2] and temperature on soil respiration in a boreal forest using δ13C as a labelling tool. Ecosyst 9:1266–1277
Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob Change Biol 4:43–54
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
De Boeck HJ, Lemmens CMHM, Vicca S, Van den Berge J, Van Dongen S, Janssens IA, Ceulemans R, Nijs I (2007) How do climate warming and species richness affect CO2 fluxes in experimental grasslands? New Phytol 175:512–522
De Boeck HJ, Lemmens CMHM, Zavalloni C, Gielen B, Malchair S, Carnol M, Merckx R, Van den Berge J, Ceulemans R, Nijs I (2008) Biomass production in experimental grasslands of different species richness during three years of climate warming. Biogeosciences 5:585–594
Díaz S (1996) Effects of elevated [CO2] at the community level mediated by root symbionts. Plant Soil 187:309–320
Edwards NT, Norby RJ (1999) Below-ground respiratory responses of sugar maple saplings to atmospheric CO2 enrichment and elevated air temperature. Plant Soil 206:85–97
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in changing environment: implications for root longevity. New Phytol 147:33–42
Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob Change Biol 10:2121–2138
European Environment Agency (2005) The European environment: state and outlook 2005. Part A, Integrated Assessment, European Environment Agency, ISBN: 92-9167776-0
Garten CT, Classen AT, Norby RJ (2009) Soil moisture surpasses elevated CO2 and temperature as a control on soil carbon dynamics in a multi-factor climate change experiment. Plant Soil 319:85–94
Gavito ME, Curtis PS, Mikkelsen TN, Jakobsen I (2000) Atmospheric CO2 and mycorrhiza effects on biomass allocation and nutrient uptake of nodulated pea (Pisum sativum) plants. J Exp Bot 51:1931–1938
Gavito ME, Curtis PS, Mikkelsen TN, Jakobsen I (2001) Interactive effects of soil temperature, atmospheric carbon dioxide and soil N on root development, biomass and nutrient uptake of winter wheat during vegetative growth. J Exp Bot 52:1913–1923
Gavito ME, Schweiger P, Jakobsen I (2003) P uptake by arbuscular mycorrhizal hyphae: effect of soil temperature and atmospheric CO2 enrichment. Glob Change Biol 9:106–116
Gavito ME, Olsson PA, Rouhier H, Medina-Penafiel A, Jakobsen I, Bago A, Azcon-Aguilar C (2005) Temperature constraints on the growth and functioning of root organ cultures with arbuscular mycorrhizal fungi. New Phytol 168:179–188
Gilmanov TG, Soussana JE, Aires L, Allard V, Ammann C, Balzarolo M, Barcza Z, Bernhofer C, Campbell CL, Cernusca A, Cescatti A, Clifton-Brown J, Dirks BOM, Dore S, Eugster W, Fuhrer J, Gimeno C, Gruenwald T, Haszpra L, Hensen A, Ibrom A, Jacobs AFG, Jones MB, Lanigan G, Laurila T, Lohila A, Manca G, Marcolla B, Nagy Z, Pilegaard K, Pinter K, Pio C, Raschi A, Rogiers N, Sanz MJ, Stefani P, Sutton M, Tuba Z, Valentini R, Williams ML, Wohlfahrt G (2007) Partitioning European grassland net ecosystem CO2 exchange into gross primary productivity and ecosystem respiration using light response function analysis. Agric Ecosyst Environ 121:93–120
Grime JP, Mackey JML, Hillier SH, Read DJ (1987) Floristic diversity in a model system using experimental microcosms. Nature 328:420–422
Hajiboland R, Aliasgharzad N, Barzeghar R (2009) Influence of arbuscular mycorrhizal fungi on uptake of Zn and P by two contrasting rice genotypes. Plant Soil Environ 55:93–100
Hartley IP, Heinemeyer A, Evans SP, Ineson P (2007a) The effect of soil warming on bulk soil vs. rhizosphere respiration. Global Change Biol 13:2654–2667
Hartley IP, Heinemeyer A, Ineson P (2007b) Effects of three years of soil warming and shading on the rate of soil respiration: substrate availability and not thermal acclimation mediates observed response. Glob Change Biol 13:1761–1770
Hawkes CV, Hartley IP, Ineson P, Fitter AH (2008) Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Glob Change Biol 14:1–10
Hebeisen T, Lüscher A, Zanetti S, Fischer BU, Hartwig UA, Frehner M, Hendrey GR, Blum H, Nösberger J (1997) The different response of Trifolium repens L. and Lolium perenne L. grassland to free air CO2 enrichment and management. Glob Change Biol 3:149–160
Heinemeyer A, Fitter AH (2004) Impact of temperature on the arbuscular mycorrhizal (AM) symbiosis: growth responses of the host plant and its AM fungal partner. J Exp Bot 55:525–534
Heinemeyer A, Ineson P, Ostle N, Fitter AH (2006) Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytol 171:159–170
Hilber U, Schüepp H, Strasser R (1998) Impact of specific groups of soil microorganisms on behaviour, bioavailability and biodegradation of pesticides, Project Report Abstract, COST-action 66, http://www.sbf.admin.ch/htm/dokumentation/publikationen/international/cost/cd2011/cost/C95.0036.html (last accessed, 30/09/2011)
Hyvönen R, Ågren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG, Kellomaki S, Lindroth A, Loustau D, Lundmark T, Norby RJ, Oren R, Pilegaard K, Ryan MG, Sigurdsson BD, Stromgren M, van Oijen M, Wallin G (2007) The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173:463–480
Jakobsen J, Smith SE, Smith FA (2002) Function and diversity of arbuscular mycorrhizae in carbon and mineral nutrition. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 75–92
Janssens IA, Pilegaard K (2003) Large seasonal changes in Q10 of soil respiration in a beech forest. Glob Change Biol 9:911–918
Kandeler E, Tscherko D, Bardgett RD, Hobbs PJ, Kompichler C, Jones TH (1998) The response of soil microorganism and roots to elevated CO2 and temperature in a terrestrial model ecosystem. Plant Soil 202:251–262
King JS, Thomas RS, Strain BR (1997) Morphology and tissue quality of seedling root systems of Pinus tadea and Pinus ponderosa as affected by varying CO2, temperature and nitrogen. Plant Soil 195:107–119
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS® for mixed models, 2nd edn. SAS Institute Inc, Cary
Loveys BR, Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK (2003) Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob Change Biol 9:895–910
Luo Y, Zhou X (2006) Soil respiration and the environment. Chapter 5: controlling farctors. Academic, Burlington, pp 79–105
Luo Y, Jackson RB, Field CB, Mooney HA (1996) Elevated CO2 increases belowground respiration in California grasslands. Oecologia 108:130–137
Luo YQ, Wan SQ, Hui DF, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413:622–625
Luo Y, Hui D, Zhang D (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87:53–63
Meenakshi SN, Manian S, Jeyaramraja PR (2007) Influence of Azoxystrobin and Difenoconazole on N2-fixing and antagonistic organisms. Pest Technol 1:139–144
Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Ahrens T, Marrisseau S (2002) Soil warming and carbon-cycle feedbacks to the climate system. Science 298:2173–2176
Morgan JA, Pataki DE, Korner C, Clark H, Del Grosso SJ, Grunzweig JM, Knapp AK, Mosier AR, Newton PCD, Niklaus PA, Nippert JB, Nowak RS, Parton WJ, Polley HW, Shaw MR (2004) Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25
Moyano FE, Kutsch WL, Schultz E-D (2007) Response of mycorrhizal, rhizosphere and soil basal respiration to temperature and photosynthesis in a barley field. Soil Biol Biochem 39:843–853
Nagy Z, Pinter K, Czobel S, Balogh J, Horvath L, Foti S, Barcza Z, Weidinger T, Csintalan Z, Dinh NQ, Grosz B, Tuba Z (2007) The carbon budget of semi-arid grassland in a wet and a dry year in Hungary. Agric Ecosyst Environ 121:21–29
Neumann E, George E (2004) Colonization with the arbuscular mycorrhiza fungus Glomus mosseae (Nicol. & Gerd) enhanced phosphorus uptake from dry soil in Sorghum bicolor (L.). Plant Cell Environ 261:245–255
Newman EI (1988) Mycorrhizal links between plants: their functioning and ecological significance. Ecol Res 18:243–270
Nijs I, Roy J, Salager JL, Fabreguettes J (2000) Elevated CO2 alters carbon fluxes in early successional Mediterranean ecosystems. Glob Change Biol 6:981–994
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2—do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol 162:253–280
Pajari B (1995) Soil respiration in a poor upland site of Scots pine stand subjected to elevated temperatures and atmospheric carbon concentration. Plant Soil 168–169:563–570
Pendall E, Mosier AR, Morgan JA (2004) Rhizodeposition stimulated by elevated CO2 in a semiarid grassland. New Phytol 162:447–458
Pendall E, Rustad L, Schimel J (2008) Towards a predictive understanding of belowground process responses to climate change: have we moved any closer? Funct Ecol 22:937–940
Pendall E, Osanai Y, Williams A, Hovenden MA (2011) Soil C storage under simulated climate change is mediated by plant functional type. Glob Change Biol 17:505–514
Plenchette C, Morel C (1996) External phosphorus requirement of mycorrhizal and non-mycorrhizal barley and soybean plants. Biol Fertil Soils 21:303–308
Poorter H (1998) Do slow-growing species and nutrient-stressed plants respond relatively strongly to elevated CO2? Glob Change Biol 4:693–697
Pregitzer KS, Laskowski MJ, Burton AJ, Lessard VC, Zak DR (1998) Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol 18:665–670
Rillig MC, Field CB, Allen MF (1999a) Soil biota responses to long-term atmospheric CO2 enrichment in two California annual grasslands. Oecologia 119:572–577
Rillig MC, Wright SF, Allen MF, Field CB (1999b) Rise in carbon dioxide changes in soil structure. Nature 400:628
Rillig MC, Treseder KK, Allen MF (2002a) Global change and mycorrhizal fungi. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 135–160
Rillig MC, Wright SF, Shaw MR, Field CB (2002b) Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 97:52–58
Rogers HH, Runion GB (1994) Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ Pollut 83:155–189
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Saleska SR, Harte J, Torn MS (1999) The effect of experimental ecosystem warming on CO2 fluxes in a montane meadow. Glob Change Biol 5:125–141
Sanders IR, Streitwolf-Engel R, van der Heijden MGA, Boller T, Wiemken A (1998) Increased allocation to external hyphae of arbuscular mycorrhizal fungi under CO2 enrichment. Oecologia 117:496–503
Scheel KC (1936) Colorimetric determination of phosphoric acid in fertilizer with the Pulfrich photometer. Z Anal Chem 105:256–269
Scheublin TR, van Logtestijn RSP, van der Heijden MGA (2007) Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J Ecol 95:631–638
Simard SW, Durall DM, Jones MD (2002) Carbon and nutrient fluxes within and between mycorrhizal plants. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 33–61
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic, London
Soussana JF, Hartwig UA (1996) The effects of elevated CO2 on symbiotic N2 fixation: a link between the carbon and nitrogen cycles in grassland ecosystems. Plant Soil 187:321–332
Soussana JE, Casella E, Loiseau P (1996) Long-term effects of CO2 enrichment and temperature increase on a temperate grass sward. II. Plant nitrogen budgets and root fractions. Plant Soil 182:101–114
Staddon PL, Fitter AH, Robinson D (1999) Effects of mycorrhizal colonization and elevated atmospheric carbon dioxide on carbon fixation and below-ground carbon partitioning in Plantago lanceolata. J Exp Bot 50:853–860
Staddon PL, Gregersen R, Jakobsen I (2004) The response of two Glomus mycorrhizal fungi and a fine endophyte to elevated atmospheric CO2, soil warming and drought. Glob Change Biol 10:1909–1921
Tamiya H (1951) Some theoretical notes on the kinetics of algal growth. Bot Mag 6:167–173
Tingey DT, Lee EH, Waschmann R, Johnson MG, Rygiewicz PT (2006) Does soil CO2 efflux acclimatize to elevated temperature and CO2 during long-term treatment of Douglas-fir seedlings? New Phytol 170:107–118
Tjoelker MG, Oleksyn J, Reich PB (1998) Seedlings of five boreal tree species differ in acclimation of net photosynthesis to elevated CO2 and temperature. Tree Physiol 18:715–726
van der Heijden MGA, Boller T, Wiemken A, Sanders IR (1998) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
Vicca S, Serrano-Ortiz P, De Boeck HJ, Lemmens CMHM, Nijs I, Ceulemans R, Kowalski AS, Janssens IA (2007) Effects of climate warming and declining species richness in grassland model ecosystems: acclimation of CO2 fluxes. Biogeosciences 4:27–36
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Volder A, Gifford RM, Evans JR (2007) Effects of elevated atmospheric CO2, cutting frequency, and differential day/night atmospheric warming on root growth and turnover of Phalaris swards. Glob Change Biol 13:1040–1052
Wan S, Norby RJ, Pregitzer KS, Ledford J, O’Neil EG (2004) CO2 enrichment and warming of the atmosphere enhance both productivity and mortality of maple tree fine roots. New Phytol 162:437–446
Wan S, Norby RJ, Ledford J, Weltzin JF (2007) Responses of soil respiration to elevated CO2 air warming, and changing soil water availability in a model old-field grassland. Glob Change Biol 13:2411–2424
Wythers KR, Reich PB, Tjoelker MG, Bolstad PB (2005) Foliar respiration acclimation to temperature and temperature variable Q(10) alter ecosystem carbon balance. Glob Change Biol 11:435–449
Zabinski CA, Quinn L, Callaway RM (2002) Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Funct Ecol 16:758–765
Zak DR, Pregitzer KS, King JS, Holmes WE (2000) Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis. New Phytol 147:201–222
Acknowledgements
This research was funded by the Belgian Science Policy Service (BELSPO, contract # SD/BD/05A) as part of the MYCARBIO project. C. Zavalloni was a beneficiary of a Marie Curie International Reintegration Grant (contract MIRG-CT-2005-031109), which partially financed this study. S. Vicca held a grant from the Institute for Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). H. Dupré de Boulois was a beneficiary of a “Chargé de Recherches” grant from the FNRS (Belgium). We thank B. Gielen and J. Van den Berge for their help during the set-up of the experiment, N. Calluy, K. Crous and F. Kockelbergh for technical assistance, P. Stevanato and S.H. Fu for their help with the root analyses, and K. Hufkens and K. Naudts for field assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katja Klumpp.
Electronic supplementarymaterial
Below is the link to the electronic supplementary material.
Figure 6
Soil respiration (Rsoil) as a function of soil temperature in grassland communities exposed to ambient temperature and 375 ppm of CO2 (Amb, figs. a–e) or ambient temperature +3°C and 620 ppm of CO2 (T+CO 2, figs f–j) measured at the end of the growing season (November). Individual regressions were fitted for each community (replication) in each climate. Dots represent measured values and lines represent the regression fitted with equation 2. (DOC 46 kb)
Figure 7
Experimental design of the 10 climate-controlled chambers. Communities in chambers with odd numbers were exposed to ambient Tair and 375 ppm CO2 (Amb), while the ones in chambers with even number were exposed to ambient Tair +3°C and 620 ppm CO2 (future climate, T+CO 2). Communities inside the chambers in green were used for the experiment. Inside each container, the 18 plants were arranged with the scheme depicted in the example above. At transplanting, soil respiration chambers were installed on the bare soil between plants. (DOC 414 kb)
Figure 8
Inside view of a climate-controlled chamber with established grasslands communities. (DOC 724 kb)
Rights and permissions
About this article
Cite this article
Zavalloni, C., Vicca, S., Büscher, M. et al. Exposure to warming and CO2 enrichment promotes greater above-ground biomass, nitrogen, phosphorus and arbuscular mycorrhizal colonization in newly established grasslands. Plant Soil 359, 121–136 (2012). https://doi.org/10.1007/s11104-012-1190-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1190-y