Abstract
Key message
GhDRP1 acts as a negatively regulator to participate in response to drought stress possibly by modulating ABA signaling pathway and flavonoid biosynthesis pathway which affects stomata movement and thus water loss, ROS scavenging enzymes, and proline accumulation in cotton.
Abstract
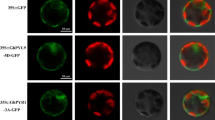
Type-2C protein phosphatases (PP2C) may play important roles in plant stress signal transduction. Here, we show the evidence that a cotton PP2C protein GhDRP1 participates in plant response to drought stress. GhDRP1 gene encodes an active type-2C protein phosphatase (PP2C) and its expression is significantly induced in cotton by drought stress. Compared with wild type, the GhDRP1 overexpression (OE) transgenic cotton and Arabidopsis displayed reduced drought tolerance, whereas GhDRP1-silenced (RNAi) cotton showed enhanced drought tolerance. Under drought stress, malondialdehyde content was lower, whereas superoxide dismutase and peroxidase activities, proline content, stomata closure and relative water content were higher in GhDRP1 RNAi plants compared with those in wild type. In contrast, GhDRP1 OE plants showed the opposite phenotype under the same conditions. Expression levels of some stress-related and flavonoid biosynthesis-related genes were altered in GhDRP1 transgenic plants under drought stress. Additionally, GhDRP1 protein could interact with other proteins such as PYLs, SNF1-related protein kinase and GLK1-like protein. Collectively, these data suggest that GhDRP1 participates in plant response to drought stress possibly by modulating ABA signaling pathway and flavonoid biosynthesis pathway which affects stomata movement and thus water loss, ROS scavenging enzymes, and proline accumulation in cotton.








Similar content being viewed by others
References
Allen RD, Webb RP, Schake SA (1997) Use of transgenic plants to study antioxidant defenses. Free Radic Biol Med 23:473–479
Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI (1999) Arabidopsis abi1 ± 1 and abi2 ± 1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11:1785–1798
Antoni R, Gonzalez-Guzman M, Rodriguez L, Rodrigues A, Pizzio GA, Rodriguez PL (2012) Selective inhibition of clade A phosphatases type 2C by PYR/PYL/RCAR abscisic acid receptors. Plant Physiol 158:970–980
Asif MA, Zafar Y, Iqbal J, Iqbal MM, Rashid U, Ali GM, Arif A, Nazir F (2011) Enhanced expression of AtNHX1, in Transgenic Groundnut (Arachis hypogaea L.) improves salt and drought tolerence. Mol Biotechnol 49(3):250–256
Baek W, Lim CW, Lee SC (2018) A DEAD-box RNA helicase, RH8, is critical for regulation of ABA signalling and the drought stress response via inhibition of PP2CA activity. Plant Cell Environ 41:1593–1604
Bharti P, Mahajan M, Vishwakarma AK, Bhardwaj J, Yadav SK (2015) AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco. J Exp Bot 66:5959–5969
Bhaskara GB, Nguyen TT, Verslues PE (2012) Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol 160:379–395
Bianchi MW, Roux C, Vartanian N (2002) Drought regulation of GST8, encoding the Arabidopsis homologue of ParC/Nt107 glutathione transferase/peroxidase. Physiol Plant 116:96–105
Bors W, Heller W, Michel C, Saran M (1990) Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol 186:343–355
Che´rel I, Michard E, Platet N, Mouline K, Alcon C, Sentenac H, Thibaud J (2002) Physical and functional interaction of the Arabidopsis K(+) channel AKT2 and phosphatase AtPP2CA. Plant Cell 14:1133–1146
Chen H, An R, Tang JH, Cui XH, Hao FS, Chen J, Wang XC (2007) Over-expression of a vacuolar Na+/H+ anti-porter gene improves salt tolerance in an upland rice. Mol Breeding 19(3):215–225
Chen Y, Liu ZH, Feng L, Zheng Y, Li DD, Li XB (2013) Genome-wide functional analysis of cotton (Gossypium hirsutum) in response to drought. PLoS ONE 8:e80879
Chen Y, Feng L, Wei N, Liu ZH, Li XB (2017) Overexpression of cotton PYL genes in Arabidopsis enhances the transgenic plant tolerance to drought stress. Plant Physiol Biochem 115:229–238
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Dupeux F, Santiago J, Betz K, Twycross J, Park SY, Rodriguez L, Gonzalez-Guzman M, Jensen MR, Krasnogor N, Blackledge M, Holdsworth M, Cutler SR, Rodriguez PL, Márquez JA (2011) A thermodynamic switch modulates abscisic acid receptor sensitivity. EMBO J 30:4171–4184
Feng F, Qi W, Lv Y, Yan S, Xu L, Yang W, Yuan Y, Chen Y, Zhao H, Song R (2018) OPAQUE11 is a central hub of the regulatory network for maize endosperm development and nutrient metabolism. Plant Cell 30:375–396
Finkelstein RR, Somerville CR (1990) Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlap** subsets of ABA responses. Plant Physiol 94:1172–1179
Finkelstein R, Reeves W, Ariizumi T, Steber C (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59:387–415
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Fuchs S, Grill E, Meskiene I, Schweighofer A (2013) Type 2C protein phosphatases in plants. FEBS J 280:681–693
Fuchs S, Tischer SV, Wunschel C, Christmann A, Grill E (2014) Abscisic acid sensor RCAR7/PYL13, specific regulator of protein phosphatase coreceptors. Proc Natl Acad Sci USA 111:5741–5746
Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009a) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462:660–664
Fujita Y, Nakashima K, Yoshida T, Katagiri T, Kidokoro S, Kanamori N, Umezawa T, Fujita M, Maruyama K, Ishiyama K, Kobayashi M, Nakasone S, Yamada K, Ito T, Shinozaki K, Yamaguchi-Shinozaki K (2009b) Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol 50:2123–2132
Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KA, Grill E, Romeis T, Hedrich R (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107:8023–8028
Hao Q, Yin P, Yan C, Yuan X, Li W, Zhang Z, Liu L, Wang J, Yan N (2010) Functional mechanism of the abscisic acid agonist pyrabactin. J Biol Chem 285:28946–28952
Harshavardhan VT, Van Son L, Seiler C, Junker A, Weigelt-Fischer K, Klukas C, Altmann T, Sreenivasulu N, Bäumlein H, Kuhlmann M (2014) AtRD22 and AtUSPL1, members of the plant-specific BURP domain family involved in Arabidopsis thaliana drought tolerance. PLoS ONE 9:e110065
Hou S, Zhu G, Li Y, Li W, Fu J, Niu E, Li L, Zhang D, Guo W (2018) Genome-wide association studies reveal genetic variation and candidate genes of drought stress related traits in cotton (Gossypium hirsutum L.). Front Plant Sci 9:1276
Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24:1695–1708
Joshi-Saha A, Valon C, Leung J (2011) Abscisic acid signal off the STARting block. Mol Plant 4:562–580
Kim TH, Boöhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2 + signaling. Annu Rev Plant Biol 61:561–591
Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61:377–383
Le Martret B, Poage M, Shiel K, Nugent GD, Dix PJ (2011) Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J 9:661–673
Lee C, Yong H, Cheong YH, Kim KN, Girdhar K (2010) Protein kinases and phosphatases for stress signal transduction in plants. In: Pareek A, Sopory SK, Bohnert HJ, Govindjee (eds) Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. Springer, Amsterdam, pp 123–164
Leung J, Merlot S, Giraudat J (1997) The Arabidopsis abscisic acid-insensitive 2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9:759–771
Li XB, Cai L, Cheng NH, Liu JW (2002) Molecular characterization of the cotton GhTUB1 gene that is preferentially expressed in fiber. Plant Physiol 130:666–674
Li XB, Fan XP, Wang XL, Cai L, Yang WC (2005) The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. Plant Cell 17:859–875
Li X, Li G, Li Y, Kong X, Zhang L, Wang J, Li X, Yang Y (2018) ABA receptor subfamily III enhances abscisic acid sensitivity and improves the drought tolerance of Arabidopsis. Int J Mol Sci 19(7):1938
Lowe J, Cha H, Lee MO, Mazur SJ, Appella E, Fornace AJ (2012) Regulation of the Wip1 phosphatase and its effects on the stress response. Front Biosci 17:1480–1498
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324:1064–1068
Ma LF, Li Y, Chen Y, Li XB (2016) Improved drought and salt tolerance of Arabidopsis thaliana by ectopic expression of a cotton (Gossypium hirsutum) CBF gene. Plant Cell Tiss Org 124:583–598
Malinova I, Kunz HH, Alseekh S, Herbst K, Fernie AR, Gierth M, Fettke J (2014) Reduction of the cytosolic phosphoglucomutase in Arabidopsis reveals impact on plant growth, seed and root development, and carbohydrate partitioning. PLoS One 9:e112468
Matus JT, Poupin MJ, Cañón P, Bordeu E, Alcalde JA, Arce-Johnson P (2010) Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol Biol 72:607–620
Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25:295–303
Min L, Li YY, Hu Q, Zhu L, Gao W, Wu Y, Ding Y, Liu S, Yang X, Zhang X (2014) Sugar and auxin signaling pathways respond to high temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol 164:1293–1308
Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, Cutler SR (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324:1068–1071
Pizzio GA, Rodriguez L, Antoni R, Gonzalez-Guzman M, Yunta C, Merilo E, Kollist H, Albert A, Rodriguez PL (2013) The PYL4 A194T mutant uncovers a key role of PYR1-like4/protein phosphatase 2CA interaction for abscisic acid signaling and plant drought resistance. Plant Physiol 163:441–455
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41:1229–1234
Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez- Garcia MP, Nicolas C, Lorenzo O, Rodriguez PL (2004) Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J 37:354–369
Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park SY, Marquez JA, Cutler SR, Rodriguez PL (2009) Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J 60:575–588
Schweighofer A, Hirt H, Meskiene I (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci 9:236–243
Shazadee H, Khan N, Wang J, Wang C, Zeng J, Huang Z, Wang X (2019) Identification and expression profiling of protein phosphatases (PP2C) gene family in Gossypium hirsutum L. Int J Mol Sci 20:E1395
Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115:327–334
Singh A, Pandey GK (2012) Protein phosphatases: a genomic outlook to understand their function in plants. J Plant Biochem Biotechnol 21:100–107
Singh A, Giri J, Kapoor S, Tyagi AK, Pandey GK (2010) Protein phosphatase complement in rice: genome-wide identification and transcriptional analysis under abiotic stress conditions and reproductive development. BMC Genom 11:435
Singh DP, Prabha R, Meena KK, Sharma L, Sharma AK (2014) Induced accumulation of polyphenolics and flavonoids in cyanobacteria under salt stress protects organisms through enhanced antioxidant activity. Am J Plant Sci 5:726–735
Singh R, Pandey N, Naskar J, Shirke PA (2015) Physiological performance and differential expression profiling of genes associated with drought tolerance in contrasting varieties of two Gossypium species. Protoplasma 252:423–438
Singh A, Pandey A, Srivastava AK, Tran LP, Pandey GK (2016) Plant protein phosphatases 2C: from genomic diversity to functional multiplicity and importance in stress management. Crit Rev Biotechnol 36:1023–1035
Smirnoff N (1993) The role of active oxygen in the response to water deficit and desiccation. New Phytol 125:27–58
Sun L, Wang YP, Chen P, Ren J, Ji K, Li Q, Li P, Dai SJ, Leng P (2011) Transcriptional regulation of SlPYL, SlPP2C, and SlSnRK2 gene families encoding ABA signal core components during tomato fruit development and drought stress. J Exp Bot 62:5659–5669
Szechyńska-Hebda M, Czarnocka W, Hebda M, Bernacki MJ, Karpiński S (2016) PAD4, LSD1 and EDS1 regulate drought tolerance, plant biomass production, and cell wall properties. Plant Cell Rep 35:527–539
Szostkiewicz I, Richter K, Kepka M, Demmel S, Ma Y, Korte A, Assaad FF, Christmann A, Grill E (2010) Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J 61:25–35
Tambussi EA, Casadesus J, Munné-Bosch S, Araus JL (2002) Photoprotection in water-stressed plants of durum wheat (Triticum turgidum var. durum): changes in chlorophyll fluorescence, spectral signature and photosynthetic pigments. Funct Plant Biol 29:35–44
Tischer SV, Wunschel C, Papacek M, Kleigrewe K, Hofmann T, Christmann A, Grill E (2017) Combinatorial interaction network of abscisic acid receptors and coreceptors from Arabidopsis thaliana. Proc Natl Acad Sci USA 114:10280–10285
Uhrig RG, Labandera AM, Moorhead GB (2013) Arabidopsis PPP family of serine/threonine protein phosphatases: many targets but few engines. Trends Plant Sci 18:505–513
Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Nat Acad Sci USA 106:17588–17593
Van Breusegem F, Vranová E, Dat JF, Inzé D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Verslues PE, Bray EA (2006) Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J Exp Bot 57:201–212
Vranová E, Inzé D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Zeng L, Wanamaker SI, Mandal J, Xu J, Cui X, Close TJ (2005) Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol 139:822–835
Wang NN, Zhao LL, Lu R, Li Y, Li XB (2015) Cotton mitogenactivated protein kinase4 (GhMPK4) confers the transgenic Arabidopsis hypersensitivity to salt and osmotic stresses. Plant Cell Tissue Organ Cult 123:619–632
Wei K, Pan S (2014) Maize protein phosphatase gene family: identification and molecular characterization. BMC Genom 15:773
Wu LH, Zhou MQ, Shen C, Liang J, Lin J (2012) Transgenic tobacco plants expressing CbCOR15b from Capsella bursa-pastoris showen hanced accumulation and tolerance to cold. J Plant Physiol 169:1408–1416
**ang Y, Sun X, Gao S, Qin F, Dai M (2017) Deletion of an endoplasmic reticulum stress response element in a ZmPP2C-A gene facilitates drought tolerance of maize seedlings. Mol Plant 10:456–469
Xue T, Wang D, Zhang S, Ehlting J, Ni F, Jakab S, Zheng C, Zhong Y (2008) Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genom 9:550
Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281:5310–5318
You J, Zong W, Hu H, Li X, **ao J, **ong L (2014) A STRESS-RESPONSIVE NAC1-regulated protein phosphatase gene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant Physiol 166(4):2100–2114
Zhang ZT, Zhou Y, Li Y, Shao SQ, Li BY, Shi HY, Li XB (2010) Interactome analysis of the six cotton 14-3-3s that are preferentially expressed in fibres and involved in cell elongation. J Exp Bot 61:3331–3344
Zhou L, Wang NN, Kong L, Gong SY, Li Y, Li XB (2014) Molecular characterization of 26 cotton WRKY genes that are expressed differentially in tissues and are induced in seedlings under high salinity and osmotic stress. Plant Cell Tissue Organ Cult 119:141–156
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 31871667, 32001594), and the Project from the Ministry of Agriculture of China for transgenic research (Grant No. 2014ZX0800927B).
Author information
Authors and Affiliations
Contributions
X-BL and YC conceived and designed the research, YC, J-BZ, NW, Z-HL and YL performed the experiments, Y.C., Y.Z. and X.-B.L. analyzed the data, Y.C. and X.-B.L wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11103_2021_1198_MOESM2_ESM.xls
Dataset S1. Summary of the genes up-regulated in leaves of wild type (WT), GhDRP1OE and GhDRP1RNAi plants under normal and drought conditions by RNA-seq assay. Supplementary material 2 (XLS 1127.0 kb)
11103_2021_1198_MOESM3_ESM.xls
Dataset S2. Summary of the genes down-regulated in leaves of wild type (WT), GhDRP1OE and GhDRP1RNAi plants under normal and drought conditions by RNA-seq assay. Supplementary material 3 (XLS 1216.5 kb)
11103_2021_1198_MOESM4_ESM.xls
Dataset S3. List of 2096 genes that show similar expression patterns in wild type (WT), GhDRP1 OE and GhDRP1 RNAi plants under drought treatments, respectively. Supplementary material 4 (XLS 737.5 kb)
11103_2021_1198_MOESM5_ESM.xls
Dataset S4. List of 585 genes that both responded to drought stress and were affected by GhDRP1. Supplementary material 5 (XLS 432.0 kb)
11103_2021_1198_MOESM6_ESM.xls
Dataset S5. List of 25 genes that show opposite expression tendency in cotton leaves under normal conditions and drought treatments between GhDRP1 OE and RNAi transgenic cotton plants. Supplementary material 6 (XLS 37.5 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, JB., Wei, N. et al. A type-2C protein phosphatase (GhDRP1) participates in cotton (Gossypium hirsutum) response to drought stress. Plant Mol Biol 107, 499–517 (2021). https://doi.org/10.1007/s11103-021-01198-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-021-01198-w