Abstract
Objective
This study aims to establish a Flow-through Visualization Dissolution System (FVDS) that combines time-lapse macro-imaging and a flow-through cell to simultaneously elucidate dissolution and disintegration profiles.
Methods
Three cefaclor extended-release tablets (CEC-1, CEC-2, CEC-3) from different manufacturers were subjected to dissolution tests using both the US Pharmacopeia basket method and the FVDS method. Two dissolution media plans were implemented in FVDS: i) Plan I involved dissolution in pH1.0 medium for 12 h; ii) Plan II initiated dissolution in pH1.0 medium for 1 h, followed by pH6.8 phosphate buffer for 11 h. The resulting dissolution data were fitted using classic mathematical models. Pixel information was further extracted from images obtained using FVDS and plotted over time.
Results
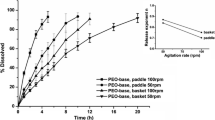
The basket method showed the cumulative dissolution of all three tablets in pH1.0, pH4.0 and water reached 80% within 6 h, but remained below 60% in the pH6.8 medium. The f2 values indicated CEC-2 was similar to CEC-1 in the pH4.0 medium, pH6.8 medium and water. Using FVDS with medium plan II, the cumulative dissolution of CEC-1 and CEC-2 reached about 80% showing similarity, while no similarity was observed between CEC-3 and CEC-1. The f2 factor of the percentage area change profiles also showed consistent results in the dissolution profile of medium plan II. However, FVDS with medium plan I cannot distinguish between CEC-2 and CEC-3.
Conclusion
FVDS offers an alternative to traditional dissolution methods by integrating imaging analysis as a complementary tool to disintegration and dissolution testing methods.








Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available in the paper and its Supplementary Information files.
References
Ligon BL. Penicillin: its discovery and early development. Semin Pediatr Infect Dis. 2004;15(1):52–7. https://doi.org/10.1053/j.spid.2004.02.001.
Nero TL, Parker MW, Morton CJ. Protein structure and computational drug discovery. Biochem Soc Trans. 2018;46(5):1367–79. https://doi.org/10.1042/bst20180202.
Vénien-Bryan C, Li Z, Vuillard L, Boutin JA. Cryo-electron microscopy and X-ray crystallography: complementary approaches to structural biology and drug discovery. Acta Crystallogr Sect F Struct Biol Commun. 2017;73(Pt 4):174–83. https://doi.org/10.1107/s2053230x17003740.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–9. https://doi.org/10.1038/s41586-021-03819-2.
Callaway E. What’s next for AlphaFold and the AI protein-folding revolution. Nature. 2022;604(7905):234–8. https://doi.org/10.1038/d41586-022-00997-5.
5 - Mathematical models of drug release. In: Bruschi ML, editor. Strategies to Modify the Drug Release from Pharmaceutical Systems: Woodhead Publishing; 2015. p. 63–86.
Amaral Silva D, Webster G, Bou-Chacra N, Löbenberg R. The significance of disintegration testing in pharmaceutical development. Dissolution Technol. 2018;25:30–8. https://doi.org/10.14227/DT250318P30.
Markl D, Zeitler JA. A review of disintegration mechanisms and measurement techniques. Pharm Res. 2017;34(5):890–917. https://doi.org/10.1007/s11095-017-2129-z.
Smrčka D, Dohnal J, Štěpánek F. Dissolution and disintegration kinetics of high-active pharmaceutical granules produced at laboratory and manufacturing scale. Eur J Pharm Biopharm. 2016;106:107–16. https://doi.org/10.1016/j.ejpb.2016.04.005.
Nickerson B, Kong A, Gerst P, Kao S. Correlation of dissolution and disintegration results for an immediate-release tablet. J Pharm Biomed Anal. 2018;150:333–40. https://doi.org/10.1016/j.jpba.2017.12.017.
Al-Gousous J, Langguth P. Oral solid dosage form disintegration testing — the forgotten test. J Pharm Sci. 2015;104(9):2664–75. https://doi.org/10.1002/jps.24303.
Chen Y, Gao Z, Duan JZ. Chapter 13 - Dissolution Testing of Solid Products. In: Qiu Y, Chen Y, Zhang GGZ, Yu L, Mantri RV, editors. Develo** solid oral dosage forms. 2nd ed. Boston: Academic Press; 2017. p. 355–80.
Dorozyński PP, Kulinowski P, Mendyk A, Młynarczyk A, Jachowicz R. Novel application of MRI technique combined with flow-through cell dissolution apparatus as supportive discriminatory test for evaluation of controlled release formulations. AAPS PharmSciTech. 2010;11(2):588–97. https://doi.org/10.1208/s12249-010-9418-8.
Gengji J, Gong T, Zhang Z, Deng L, Fu Y. Imaging techniques for studying solid dosage formulation: principles and applications. J Control Release. 2023;361:659–70. https://doi.org/10.1016/j.jconrel.2023.08.013.
Sohail Arshad M, Zafar S, Yousef B, Alyassin Y, Ali R, AlAsiri A, et al. A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Adv Drug Deliv Rev. 2021;178: 113840. https://doi.org/10.1016/j.addr.2021.113840.
Deng L, Fu Y. Seeing is believing: time-lapse macro-imaging of morphological changes of solid dosages as a teaching and research tool. Pharm Res. 2022;39(5):1019–24. https://doi.org/10.1007/s11095-022-03271-5.
SpasiĆ. influence of dissolution media composition on cefaclor release form capsules. Sci Pharm. 2010;78:610-. https://doi.org/10.3797/scipharm.cespt.8.PDD21.
USP. <711> Dissolution. https://doi.org/10.31003/USPNF_M99470_02_01. 2023. Accessed 21 Jan 2023.
USP. <1092> The dissolution procedure development and validation. https://doi.org/10.31003/USPNF_M643_05_01.2023. Accessed 21 Jan 2023.
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China (Volume 4). Bei**g: China Medical Science Press; 2020.
USP. Dissolution Methods Database. https://www.usp.org/resources/dissolution-methods-database. 2021. Accessed 17 May 2021.
Siepmann J, Peppas NA. Higuchi equation: derivation, applications, use and misuse. Int J Pharm. 2011;418(1):6–12. https://doi.org/10.1016/j.ijpharm.2011.03.051.
Dimitrovska A, Stojanoski K, Dorevski K. Kinetics of degradation of cefaclor: I. Effects of temperature, phosphate buffer, pH and copper(II) ion on the rate of degradation. Int J Pharm. 1995;115(2):175–82. https://doi.org/10.1016/0378-5173(94)00244-Y.
Quodbach J, Kleinebudde P. A critical review on tablet disintegration. Pharm Dev Technol. 2016;21(6):763–74. https://doi.org/10.3109/10837450.2015.1045618.
Desai PM, Liew CV, Heng PWS. Review of disintegrants and the disintegration phenomena. J Pharm Sci. 2016;105(9):2545–55. https://doi.org/10.1016/j.xphs.2015.12.019.
Markl D, Maclean N, Mann J, Williams H, Abbott A, Mead H, et al. Tablet disintegration performance: Effect of compression pressure and storage conditions on surface liquid absorption and swelling kinetics. Int J Pharm. 2021;601: 120382. https://doi.org/10.1016/j.ijpharm.2021.120382.
Acknowledgements
Authors are grateful for the financial support from Sichuan University (“0-to-1” project), 111 project (B18035) and the Fundamental Research Funds for the Central Universities.
Funding
Li Deng received funding support from Sichuan University (“0-to-1” project).
Author information
Authors and Affiliations
Contributions
Yichen Yang: Writing—Original Draft, Methodology, Validation, Investigation. Jiajia Gengj: Writing—Original Draft, Formal analysis. Tao Gong: Funding acquisition. Zhirong Zhang: Funding acquisition. Li Deng: Conceptualization, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (M4V 135535 KB)
Supplementary file3 (M4V 141344 KB)
Supplementary file4 (M4V 71128 KB)
Supplementary file5 (M4V 71855 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Gengji, J., Gong, T. et al. Time-Lapse Macro Imaging with Dissolution Tests for Exploring the Interrelationship Between Disintegration and Dissolution Behaviors of Solid Dosages. Pharm Res 41, 387–400 (2024). https://doi.org/10.1007/s11095-024-03655-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03655-9