Abstract
Purpose
The freezing step in lyophilization is the most determinant for the quality of biopharmaceutics. Using insulin as model of therapeutic protein, our aim was to evaluate the freezing effect in the stability and bioactivity of insulin-loaded PLGA nanoparticles. The performance of trehalose, sucrose and sorbitol as cryoprotectants was evaluated.
Methods
Cryoprotectants were co-encapsulated with insulin into PLGA nanoparticles and lyophilized using an optimized cycle with freezing at −80°C, in liquid nitrogen, or ramped cooling at −40°C. Upon lyophilization, the stability of protein structure and in vivo bioactivity were assessed.
Results
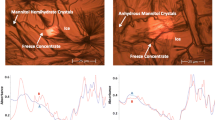
Insulin was co-encapsulated with cryoprotectants resulting in particles of 243–394 nm, zeta potential of −32 to −35 mV, and an association efficiency above 90%. The cryoprotectants were crucial to mitigate the freezing stresses and better stabilize the protein. The insulin structure maintenance was evident and close to 90%. Trehalose co-encapsulated insulin-loaded PLGA nanoparticles demonstrated enhanced hypoglycemic effect, comparatively to nanoparticles without cryoprotectant and added with trehalose, due to a superior insulin stabilization and bioactivity.
Conclusions
The freezing process may be detrimental to the structure of protein loaded into nanoparticles, with negative consequences to bioactivity. The co-encapsulation of cryoprotectants mitigated the freezing stresses with benefits to protein bioactivity.









Similar content being viewed by others
Abbreviations
- AAC:
-
Area above the curve
- AE:
-
Association efficiency
- ATR-FTIR:
-
Attenuated Total Reflectance-Fourier transform infrared spectroscopy
- CD:
-
Circular dichroism
- DSC:
-
Differential scanning calorimetry
- HPLC:
-
High performance liquid chromatography
- LC:
-
Loading capacity
- PA:
-
Pharmacological availability
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PVA:
-
Polyvinyl alcohol
- RE:
-
Retention efficiency
- SEM:
-
Scanning electron microscopy
- Tc:
-
Collapse temperature
- Tg:
-
Glass transition temperature
- Tg’:
-
Glass transition temperature of the frozen sample
- XRPD:
-
X-Ray powder diffraction
References
Danhier F, Ansorena E, Silva J, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22.
Fonte P, Araujo F, Silva C, Pereira C, Reis S, Santos HA, et al. Polymer-based nanoparticles for oral insulin delivery: revisited approaches. Biotechnol Adv. 2015;33:1342–54.
Konan Y, Gurny R, Allémann E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int J Pharm. 2002;233:239–52.
De Jaeghere F, Allemann E, Leroux J, Stevels W, Feijen J, Doelker E, et al. Formulation and lyoprotection of poly(lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharm Res. 1999;16:859–66.
Williams NA, Polli GP. The lyophilization of pharmaceuticals: a literature review. J Parenter Sci Technol. 1984;38:48–59.
Franks F. Freeze-drying of bioproducts: putting principles into practice. Eur J Pharm Biopharm. 1998;45:221–9.
Kasper JC, Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78:248–63.
Izutsu K, Fujimaki Y, Kuwabara A, Aoyagi N. Effect of counterions on the physical properties of l-arginine in frozen solutions and freeze-dried solids. Int J Pharm. 2005;301:161–9.
Heller M, Carpenter J, Randolph T. Protein formulation and lyophilization cycle design: prevention of damage due to freeze-concentration induced phase separation. Biotechnol Bioeng. 1999;63:166–74.
Kadoya S, Fujii K, Izutsu K, Yonemochi E, Terada K, Yomota C, et al. Freeze-drying of proteins with glass-forming oligosaccharide-derived sugar alcohols. Int J Pharm. 2010;389:107–13.
Searles J. Freezing and annealing phenomena in lyophilization. In: Rey L, May JC, editors. Freeze drying/lyophilization of pharmaceutical and biological products. Informa Healthcare; 2010. p. 52–81.
Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12:505–23.
Sameti M, Bohr G, Ravi Kumar MNV, Kneuer C, Bakowsky U, Nacken M, et al. Stabilisation by freeze-drying of cationically modified silica nanoparticles for gene delivery. Int J Pharm. 2003;266:51–60.
Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203:1–60.
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58:1688–713.
Fonte P, Soares S, Sousa F, Costa A, Seabra V, Reis S, et al. Stability study perspective of the effect of freeze-drying using cryoprotectants on the structure of insulin loaded into PLGA nanoparticles. Biomacromolecules. 2014;15:3753–65.
Fonte P, Araújo F, Seabra V, Reis S, van de Weert M, Sarmento B. Co-encapsulation of lyoprotectants improves the stability of protein-loaded PLGA nanoparticles upon lyophilization. Int J Pharm. 2015;496:850–62.
Sarmento B, Ribeiro A, Veiga F, Ferreira D. Development and validation of a rapid reversed-phase HPLC method for the determination of insulin from nanoparticulate systems. Biomed Chromatogr. 2006;20:898–903.
Dong A, Huang P, Caughey W. Protein secondary structures in water from second-derivative amide i infrared spectra. Biochemistry. 1990;29:3303–8.
Kendrick B, Dong A, Allison S, Manning M, Carpenter J. Quantitation of the area of overlap between second-derivative amide I infrared spectra to determine the structural similarity of a protein in different states. J Pharm Sci. 1996;85:155–8.
Maltesen M, Bjerregaard S, Hovgaard L, Havelund S, Van De Weert M. Analysis of insulin allostery in solution and solid state with FTIR. J Pharm Sci. 2009;98:3265–77.
Fonte P, Soares S, Costa A, Andrade J, Seabra V, Reis S, et al. Effect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-drying. Biomatter. 2012;2:329–39.
Matsumoto S. Proteins and sugars affecting the zeta potential and stability of dispersed vesicular globules in w/o/w emulsions. In: Nishinari K, Doi E, editors. Food hydrocolloids. US: Springer; 1993. p. 399–408.
Abdelwahed W, Degobert G, Fessi H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur J Pharm Biopharm. 2006;63:87–94.
Abdelwahed W, Degobert G, Fessi H. A pilot study of freeze drying of poly(epsilon-caprolactone) nanocapsules stabilized by poly(vinyl alcohol): formulation and process optimization. Int J Pharm. 2006;309:178–88.
Tang X, Pikal M. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21:191–200.
Pikal MJ, Shah S, Roy ML, Putman R. The secondary drying stage of freeze drying: drying kinetics as a function of temperature and chamber pressure. Int J Pharm. 1990;60:203–7.
Pikal M, Shah S. The collapse temperature in freeze drying: dependance on measurement methodology and rate of water removal from the glassy state. Int J Pharm. 1990;62:165–86.
Schwegman JJ, Carpenter JF, Nail SL. Evidence of partial unfolding of proteins at the ice/freeze-concentrate interface by infrared microscopy. J Pharm Sci. 2009;98:3239–46.
Searles JA, Carpenter JF, Randolph TW. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J Pharm Sci. 2001;90:860–71.
Bozdag S, Dillen K, Vandervoort J, Ludwig A. The effect of freeze-drying with different cryoprotectants and gamma-irradiation sterilization on the characteristics of ciprofloxacin HCl-loaded poly(D, L-lactide-glycolide) nanoparticles. J Pharm Pharmacol. 2005;57:699–707.
Liu J, Viverette T, Virgin M, Anderson M, Paresh D. A study of the impact of freezing on the lyophilization of a concentrated formulation with a high fill depth. Pharm Dev Technol. 2005;10:261–72.
Patapoff TW, Overcashier DE. The importance of freezing on lyophilization cycle development. BioPharm. 2002;15:16–21.
Webb SD, Cleland JL, Carpenter JF, Randolph TW. Effects of annealing lyophilized and spray-lyophilized formulations of recombinant human interferon-gamma. J Pharm Sci. 2003;92:715–29.
Pikal MJ, Rigsbee DR. The stability of insulin in crystalline and amorphous solids: observation of greater stability for the amorphous form. Pharm Res. 1997;14:1379–87.
Sarmento B, Ferreira D, Jorgensen L, van de Weert M. Probing insulin’s secondary structure after entrapment into alginate/chitosan nanoparticles. Eur J Pharm Biopharm. 2007;65:10–7.
Strambini GB, Gabellieri E. Proteins in frozen solutions: evidence of ice-induced partial unfolding. Biophys J. 1996;70:971–6.
Sarciaux JM, Mansour S, Hageman MJ, Nail SL. Effects of buffer composition and processing conditions on aggregation of bovine IgG during freeze-drying. J Pharm Sci. 1999;88:1354–61.
Eckhardt BM, Oeswein JQ, Bewley TA. Effect of freezing on aggregation of human growth hormone. Pharm Res. 1991;8:1360–4.
Chang BS, Kendrick BS, Carpenter JF. Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J Pharm Sci. 1996;85:1325–30.
Nema S, Avis KE. Freeze-thaw studies of a model protein, lactate dehydrogenase, in the presence of cryoprotectants. J Parenter Sci Technol. 1993;47:76–83.
Carpenter JF, Arakawa T, Crowe JH. Interactions of stabilizing additives with proteins during freeze-thawing and freeze-drying. Dev Biol Stand. 1992;74:225–38. discussion 238–229.
Griebenow K, Klibanov A. On protein denaturation in aqueous−organic mixtures but not in pure organic solvents. J Am Chem Soc. 1996;118:11695–700.
Van de Weert M, Hering J, Haris P. Fourier transform infrared spectroscopy. In: Jiskoot W, Crommelin D, editors. Methods for structural analysis of protein pharmaceuticals. AAPS Press; 2005. p. 131–166.
Jørgensen L, Vermehren C, Bjerregaard S, Froekjaer S. Secondary structure alterations in insulin and growth hormone water-in-oil emulsions. Int J Pharm. 2003;254:7–10.
Kelly S, Jess T, Price N. How to study proteins by circular dichroism. Biochim Biophys Acta. 2005;1751:119–39.
Yong Z, Yingjie D, Xueli W, **ghua X, Zhengqiang L. Conformational and bioactivity analysis of insulin: freeze-drying TBA/water co-solvent system in the presence of surfactant and sugar. Int J Pharm. 2009;371:71–81.
Bouchard M, Zurdo J, Nettleton EJ, Dobson CM, Robinson CV. Formation of insulin amyloid fibrils followed by FTIR simultaneously with CD and electron microscopy. Protein Sci. 2000;9:1960–7.
Ahmad A, Uversky VN, Hong D, Fink AL. Early events in the fibrillation of monomeric insulin. J Biol Chem. 2005;280:42669–75.
Bekard IB, Dunstan DE. Tyrosine autofluorescence as a measure of bovine insulin fibrillation. Biophys J. 2009;97:2521–31.
Falconi M, Bozzi M, Paci M, Raudino A, Purrello R, Cambria A, et al. Spectroscopic and molecular dynamics simulation studies of the interaction of insulin with glucose. Int J Biol Macromol. 2001;29:161–8.
Amidi M, Pellikaan HC, de Boer AH, Crommelin DJ, Hennink WE, Jiskoot W. Preparation and physicochemical characterization of supercritically dried insulin-loaded microparticles for pulmonary delivery. Eur J Pharm Biopharm. 2008;68:191–200.
Malik R, Roy I. Probing the mechanism of insulin aggregation during agitation. Int J Pharm. 2011;413:73–80.
Lakowicz JR. Principles of fluorescence spectroscopy. US: Springer; 2006.
Arigita C, Jiskoot W, Westdijk J, van Ingen C, Hennink WE, Crommelin DJ, et al. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine. 2004;22:629–42.
LeVine H. Thioflavine T interaction with amyloid β-sheet structures. Amyloid. 1995;2:1–6.
Maskevich AA, Stsiapura VI, Kuzmitsky VA, Kuznetsova IM, Povarova OI, Uversky VN, et al. Spectral properties of thioflavin t in solvents with different dielectric properties and in a fibril-incorporated form. J Proteome Res. 2007;6:1392–401.
Nielsen L, Khurana R, Coats A, Frokjaer S, Brange J, Vyas S, et al. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001;40:6036–46.
Jimenez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR. The protofilament structure of insulin amyloid fibrils. Proc Natl Acad Sci U S A. 2002;99:9196–201.
ACKNOWLEDGMENTS AND DISCLOSURES
Pedro Fonte and Fernanda Andrade would like to thank Fundação para a Ciência e a Tecnologia (FCT), Portugal (PTDC/SAL-FCT/104492/2008; SFRH/BD/78127/2011) and (SFRH/BD/73062/2010) for financial support. This work was also financed by European Regional Development Fund (ERDF) through the Programa Operacional Factores de Competitividade − COMPETE, by Portuguese funds through FCT in the framework of the project PEst-C/SAU/LA0002/2013, and cofinanced by North Portugal Regional Operational Programme (ON.2 − O Novo Norte) in the framework of Project SAESCTN-PIIC&DT/2011 under the National Strategic Reference Framework (NSRF). It is also acknowledged the financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 (UID/MULTI/04378/2013 - POCI/01/0145/FERDER/007728). Abbot Laboratories, Portugal is acknowledged for kindly provide the Precision Xtra® blood glucose meter and test strips. The authors also thank to Dorthe Ørbæk from the Faculty of Health and Medical Sciences, University of Copenhagen, for the XRPD experiments.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 561 kb)
Rights and permissions
About this article
Cite this article
Fonte, P., Andrade, F., Azevedo, C. et al. Effect of the Freezing Step in the Stability and Bioactivity of Protein-Loaded PLGA Nanoparticles Upon Lyophilization. Pharm Res 33, 2777–2793 (2016). https://doi.org/10.1007/s11095-016-2004-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2004-3