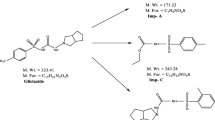
There is no stability-indicating specific related substances high-performance liquid chromatography (HPLC) method for anagliptin active pharmaceutical ingredients in the presence of its degradation products. Hence, there is a necessity to have a specific stability-indicating HPLC method for the quantification of anagliptin-related substances in the presence of its forced degradation products. The aim of the research work is to develop an innovative simple, accurate, precise, and selective HPLC method with the lesser use of organic solvents, for the quantification of anagliptin-related substances in the presence of its forced degradation products. The chromatographic separation was achieved on a YMC Pro C18 reversed-phase HPLC column (250 mm × 4.6 mm, 5 μ) with a runtime of 50 min. Mobile phase A and mobile phase B were chlorate buffer with pH 3.2 and acetonitrile respectively. The HPLC column oven temperature was set at 28°C and the photodiode array detector was set at 205 nm. The proposed test procedure has shown excellent linearity within the concentration range 0.081–6.296 μg/mL with correlation coefficient (r) about 0.9998. The limit of detection of anagliptin and related substances was observed within the range 0.052–0.106) μg/mL and the limit of quantification was observed within the range 0.156–0.317 μg/mL. The developed test procedure was successfully utilized for the quantification of related substances of anagliptin bulk drug without any interference with the degradation compounds. Hence, the test procedure can be utilized successfully in pharmaceutical organizations for the separation and quantification of anagliptin-related substances.








Similar content being viewed by others
References
International Conference on Harmonization Q1A (R2); Stability testing of new drug substances and products. ICH, IFPMA, Geneva (2003).
ICH Q1B; Photostability testing of new drug substances and products. International conference on harmonization, IFPMA, Geneva (1996).
ICH Q2 (R1); Validation of analytical procedures; text and methodology : International conference on harmonization of technical requirements for registration of pharmaceuticals for human use, IFPMA, Geneva (2005).
ICH Guideline, Validation of analytical procedures; text and methodology, in Proceedings of International Conference on Harmonization, Topic Q2 (R1), Geneva (2005).
FDA: Guidance for Industry; Validation of Analytical Procedures; Definition and Terminology Final Guidance, Silver Spring, MD, USA (2010).
FDA: CDER, Beers and Donald; Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry, Silver Spring, MD, USA (2015).
Government of India Ministry of health & family welfare; Published by Indian Pharmacopoeia Commission, 2, 503 – 728, (2014).
S. Nishio, M. Abe and H. Ito, Diabetes Metab. Syndr. Obes. Targets Ther., 8, 163 – 171 (2015).
A. B. Olokoba, O. A. Obateru, L. B. Olokoba, et al., Oman Med. J., 27(4), 269 – 273 (2014).
K. Miyanko and M. Noda, Clin. Drug. Investig., 35, 141 – 147 (2015).
C. Atsuko, T. Atsushi, M. Takeshi, et al., Cardiovasc. Diabetol., 18(158),1–8 (2019).
T. Hiroki, M. Takeshi, F. Yuichi, et al., Diabetes. Metab. Syndr. Obes., 13, 4993 – 5001(2020).
S. M. **, and S. W. Park, K. H. Yoon, et al., Diabetes. Metab. Syndr. Obes., 17(5), 511 – 515 (2015).
S. Furuta, C. Smart and A. Hackett, Xenobiotica, 43, 432 – 442 (2013).
N. Gampa, N. T. Chevala, P. Pinnamaneni, et al., Int. J. Chem. Pharm. Anal., 4(2),1 – 8 (2017).
K. D. Malleswar, B. V. Rao and S. Shylaja, Indo Am. J. Pharm. Res., 9(07), 3096 – 3101 (2019).
A. Patil and A. A. Shirkedkar, Pharm. Methods, 7(2), 127 – 131 (2016).
R. H. Majithia and J. S. Shah, Int. J. Pharmacy. Tech, 6, 7765 – 7771 (2015).
P. B. Bhatti and H. J. Panchal, World. J. Pharm. Res., 6, 956 – 973 (2017).
A. Patil and A. A. Shirkhedkar, Eurasian J. Anal. Chem., 12, 443 – 458 (2017).
S. T. Shah and D. G. Maheshwari, J. Globe. Trends Pharm. Sci., 6(4), 2925 – 2929 (2015).
P. B. Bhatti, World J. Pharm. Pharm. Sci., 6(10), 396 – 403 (2017).
S. N. Chellu, M. Malleshwararao and V. Suryanarayana, Sci. Pharm., 80, 139 – 152 (2012).
P. R. Cumar, M. Vasudevan and Deecaraman, Rasayan J. Chem., 5(2), 137 – 141 (2012).
B. Santhosha, A Ravindranath and C. Sundari, Int. Res J. Pharm. App. Sci., 2(3), 22 – 28 (2012).
P. A. Kumar, G. Aruna, K. Rajasekar, et al., Int. Bul. Drug Res., 3(5), 58 – 68 (2013).
K. S. Potdar, M. S. Kalshetti, and R. Y. Patil, Int. J. Chem. Pharm. Anal., 4(3),1–9 (2017).
M. M. Mabrouk, S. F. Hammad, F. R. Mansour, et al., Der Pharmacia Sinica, 7(2), 32 – 40 (2016).
K. Zhang, P. Ma, W. **g, et al., Asian J. Pharm. Sci., 10(2), 152 – 158 (2015).
P. Supriya, N. L. Madhavi, K. Rohith, et al., Asian J. Pharm. Clin. Res., 9(1), 282 – 287 (2016).
K. Y. Kavitha, G. Geetha, R. Hariprasad, et al., J. Chem. & Pharm. Res., 5(1), 230 – 235 (2013).
A. B. Loni, and M. R. Ghante, Der Pharma Chemica, 4(3), 854 – 859 (2012).
N. T. Lamie and M. A. Mahrouse, Spectrochim. Acta A Mol. Biomol. Spectrosc., 204, 743 – 747 (2018).
K. Sharma and A. Parle, Int. J. Pharm. Res. Rev., 4(11), 35 – 42 (2015).
P. B. Deshpande and S. R. Butle, Eurasian J. Anal. Chem., 12(4), 325 – 335 (2017).
R. H. Majithia and A. Khodadiya, Int. J. Pharm. Sci. Res., 12(1), 292 – 297 (2021).
Acknowledgements
The authors are thankful to Lupin Pharmaceutical Ltd (Tarapur, India) for providing gratis samples for the present studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chavan, R.S., Ahad, A., Phase, R. et al. Development and Validation of a Stability-Indicating Related Substances RP-HPLC Method for Anagliptin and its Degradation Products. Pharm Chem J 57, 1314–1322 (2023). https://doi.org/10.1007/s11094-024-03040-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03040-1