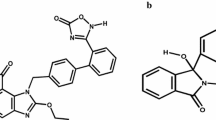
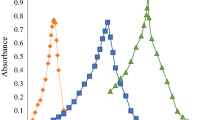
This work was aimed at the development of response surface optimised bioanalytical stability indicating the LC-UV method for the estimation of drugs such as Emtricitabine and Efavirenz. Current scenario of analysis states the need for development of a method that is sustainable and has wider applications in pharmacokinetics and bio equivalence studies. So to reduce the chances of method failure in analysis of low concentration analytes with different pH and pKa values from a biological matrix, a conceptual and strategically investigated method using PQRI database for column selection, Ishikawa cause and effect fish bone diagram for risk ranking of chromatographic parameters and screening design (Taguchi design) to filter most influential method parameters and attributes were applied for develo** a method to determine Emtricitabine and Efavirenz in presence of internal standard from bulk in human plasma sample. The optimization of variables was accomplished using boxbehnken design and the points with derringer’s desirability index 1 from experimental design were executed by obeying the chromatographic parameters stated. The percentage bias of these responses was compared to laboratory data and the result indicated % variation to be less than 5%. The method was validated for all other parameters according to ICH guidelines and variation of all parameters was reported to be less than 2% RSD.









Similar content being viewed by others
References
O. L. Bryan-Marrugo, J. Ramos-Jiménez, H. Barrera-Saldaña, et al., Med. Univ., 17, 165 – 174 (2015).
C. Z. Matthews, E. J. Woolf, R. S. Mazenko, and H. Haddix-Wiener, J. Pharm. Biomed. Anal., 28, 925 – 934 (2002).
O. D. S. Viana, F. Patrícia, M. Medeiros, et al., Brazil. J. Pharm. Sci., 47, 97 – 102 (2011).
B. N. Patel, Pharm. Methods, 3, 73 – 78 (2012).
A. L. R. D. Psrchnp Varma, Indian J. Pharm. Pharmacol., 1, 1 – 17 (2014).
R. Sharma and P. Gupta, Eurasian J. Chem., 4, 276 – 284 (2009).
P. S. Devrukhakar, R. Borkar, N. Shastri, and K. V. Surendranath, ISRN Chromatogr., 2013, 1 – 8 (2013).
S. Nadig, J. T. Jacob, I. Bhat, and V. Kishoreraju, Int. J. Res. Pharm. Sci., 4, 391 – 396 (2013).
Rao A. Srinivasa, N. G. Kumar, K. Srilekha, and Kumari N. Aruna, Int. J. Adv. Res. Sci. Eng., 5(05), 188 – 200 (2016).
D. Schlossberg, R. Samuel, EMTRIVA (Emtricitabine), in: Antibiotics Manual: A Guide to Commonly Used Antimicrobials, John Wiley and Sons (2017), pp. 141 – 142; https: https://doi.org/10.1002/9781119220787.ch64.
A. Ramaswamy, A. Smith, and A. Gnana, Arab. J. Chem., 11, 275 – 281 (2018).
N. A. Raju, J. V. Rao, and K. V. Prakash, Orient. J. Chem., 24, 645 – 650 (2008).
R. Nirogi, G. Bhyrapuneni, V. Kandikere, et al., Biomed. Chromatogr., 23, 371 – 381 (2009).
R. K. Mishra, N. Chaubey, J. R. Patel, et al., Int. J. Appl. Pharm., 12, 41 – 50 (2020).
S. Bonde, C. G. Bonde, B. Prabhakar, Microchem. J., 149, 103982 (2019).
P. Kumar, N. Rao, T. Cecchi, et al., J. Pharm. Biomed. Anal., 147, 590 – 611 (2018).
Y. Huang, H. **ao, Y. Liu, et al., Chem. Biol. Drug Design, 93(1), 29 – 37 (2019); https: //doi.org/https://doi.org/10.1111/cbdd.13375
B. Bidlingmeyer, C. C. C. P. F. Richard, P. Koerner, et al., Pharmacopoeial Forum, 31, 637 – 645 (2005).
G. C. Slack adn N. H. Snow, Sep. Sci. Technol., 8, 237 – 268 (2007).
S. L. C. Ferreira, R. E. Bruns, H. S. Ferreira, et al., Anal. Chim. Acta, 597, 179 – 186 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanumuri, R.T., Kavuri, N.R. & Sankranthi, Y. Response Surface Optimised Bioanalytical Stability Indicating LC-UV Method for the Estimation of Emtricitabine and Efavirenz with Internal Standard. Pharm Chem J 57, 608–619 (2023). https://doi.org/10.1007/s11094-023-02926-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02926-w