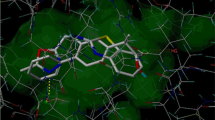
The reaction of 4-chlorobenzohydrazide 1 with carbon disulphide in the presence of methanolic potassium hydroxide afforded 4-chloro potassium dithiocarbohydrazide 2. The intermediate 2 reacts with a solution of hydrazine hydrate in water gave 4-amino-5(4-chlorophenyl)-4H-[1,2,4]-triazole-3-thiol 3. Condensation of 3 with various substituted phenacyl bromide in alcohol afforded series of 1,2,4-triazolo-[3,4-b][1,3,4]thiadiazine analogs 4a–4l bearing 4-chlorophenyl moiety. The structures of newly synthesized compounds were characterized by IR, 1H NMR, 13C NMR and mass spectroscopy techniques. All synthesized compounds 4a–4l were screened for anti-inflammatory activity by carrageenan induced paw edema using albino Wistar rats. In addition, molecular docking study was performed for 4a—4l using prostaglandin D2 synthase (PGDS) enzyme receptor binding pocket. Compounds 4l and 4b exhibited significant anti-inflammatory activity (P < 0.01), whereas compounds 4b, 4f, and 4k unveiled good anti-inflammatory activity (P < 0.05) when compared with standard drug indomethacin.






Similar content being viewed by others
References
C. Cabrele and O. Reiser, J. Org. Chem., 81(21), 10109 – 10125 (2016).
R. L. Nicolas, B. Eduardo and G. F. Vicente, Eur. J. Org. Chem., 3, 484 – 93 (2010).
A. Savateev, C. Liedel, S. Troger-Muller, et al., Chem. Commun., 53(73), 10192 (2017).
P. Deepa and D. Thirumeignanam, J. Mol. Graph. Model., 97, 107553 (2020).
P. Karegoudar, D. J. Prasad, M. Ashok, et al., Eur. J. Med. Chem., 43(4), 808 – 815 (2008).
V. S. Palekar, A. J. Damle and S. R. Shukla, Eur. J. Med. Chem., 44(12), 5112 – 5116 (2009).
Z. Marom, J. H. Shelhamer, and M. Kaliner, J. Clin. Investig., 67(6), 1695 – 1702 (1981).
F. Cipollone, G. Cicolini and M. Bucci, Pharmacol. Ther., 118(2), 161 – 180 (2008).
Z. L. Huang, Y. Urade and O. Hayaishi, Curr. Top. Med. Chem., 11(8), 1047 – 1057 (2011).
U. H. Gandhi, N. Kaushal, K. C. Ravindra, et al., Int. J. Biol. Chem., 286(31), 27471 – 27482 (2011).
T. Nakamura, S. Maeda, K. Horiguchi, et al., Nat. Commun., 6(1), 1 – 10 (2015).
W. S. Powell, Clin. Sci., 135(16), 1945 – 1980 (2021).
M. Hohwy, L. Spadola, B. Lundquist, et al., J. Med. Chem., 51(7), 2178 – 2186 (2008).
K. Yamamoto, A. Higashiura, M. Suzuki, et al., Biophys. Res. Commun., 492(2), 166 – 171 (2017).
C. A. Winter, E. A. Risley and G. W. Nussal, Proc. Soc. Exp. Biol. Med., 111(3), 544 – 547 (1962).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karande, N.A., Rathi, L.G., Kamble, K.S. et al. Synthesis, Anti-Inflammatory Activity and Molecular Docking Studies of New 1,2,4-Triazolo[3,4-b][1,3,4]Thiadiazine Derivatives. Pharm Chem J 57, 234–242 (2023). https://doi.org/10.1007/s11094-023-02873-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02873-6