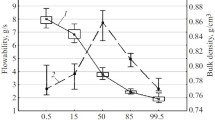
The influence of the type and ratio of co-process fillers and lubricating excipients on the properties of the final dosage form was studied during the development of the composition and technology for GK-2 [bis-(N-monosuccinyl-L-glutamyl-L-lysine hexamethyleneamide] tablets dispersed in the oral cavity. The pharmaceutical and technological properties, including the degree of flowability, bulk density under compaction, mass loss on drying, coefficient of compressibility (Carr’s index), tablet crushing strength, disintegration of tablets, and wear resistance, were evaluated to assess the nature and degree of influence of all composition factors. Analysis of variance considering the particular factors and their averages for all indicators was used to assess the degree of their influence. In addition, the determination coefficients were analyzed for each parameter, excluding parameters with low values from further studies.




Similar content being viewed by others
References
P. Yu. Povarnina, O. N. Vorontsova, T. A. Gudasheva, et al., Acta Nat. (Russian version), 5(3), 48 – 52 (2013).
P. Yu. Povarnina, T. A. Gudasheva, O. N. Vorontsova, et al., Eksp. Klin. Farmakol., 75(9), 15 – 20 (2012).
S. B. Seredenin, D. N. Silachev, T. A. Gudasheva, et al., Byull. Eksp. Biol. Med., 151(5), 518 – 519 (2011).
E. V. Blynskaya, S. V. Tishkov, K. V. Alekseev, et al., Vestn. VGU, Ser. Khim. Biol. Farm., No. 1, 117 – 126 (2019).
E. V. Blynskaya, S. V. Tishkov, K. V. Alekseev, et al., Vopr. Obespecheniya Kach. Lek. Sredstv, No. 3, 18 – 25 (2019).
E. V. Blynskaya, S. V. Tishkov, K. V. Alekseev, et al., Biofarm. Zh., 11(2), 22 – 29 (2019).
E. Blynskaya, S. Tishkov, K. Alekseev, et al., Int. J. Pharm. Res., Suppl. Iss. No. 1, 925 – 940 (2020).
A. Ganeshpurkar, V. Pandey, S. Asati, et al., Experimental Design and Analysis of Variance, Dosage Form Design Parameters, Academic Press, (2018), pp. 281 – 301.
G. A. Lewis, D. Mathieu, and R. Phan-Tan-Luu, Pharmaceutical Experimental Design, CRC Press, Boca Raton (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 10, pp. 35 – 41, October, 2021.
Rights and permissions
About this article
Cite this article
Tishkov, S.V., Blynskaya, E.V., Alekseev, K.V. et al. Use of Two-Factor Dispersion Analysis for Studying the Pharmaceutical and Technological Properties of Tablets of GK-2 – bis-(N-Monosuccinyl-L-Glutamyl-L-Lysine) Hexamethyleneamide, Dispersed in the Oral Cavity. Pharm Chem J 55, 1096–1102 (2022). https://doi.org/10.1007/s11094-021-02542-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02542-6