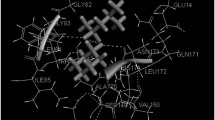
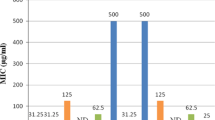
A new series of tetraoxanes were developed and screened for in vitro antimalarial activity against chloroquine sensitive strains of Plasmodium falciparum (3D7 and RKL-2) and chloroquine resistant strain of P. falciparum (RKL-9). Among the synthesized derivatives, few compounds showed mild to moderate activity against the parasites as compared to a standard drug. The test results revealed two compounds, 5a (3,3,6-trimethyl-1,2,4,5-tetraoxane) and 5k (3,3-dimethyl-6,6-diphenyl-1,2,4,5-tetraoxane) possessing significant activity against chloroquine sensitive 3D7 strain (IC50 = 1.953 ± 0.020 μg/mL) and RKL-2 strain (IC50 = 3.906 ± 0.010 μg/mL). At the same time, only compound 5j (3-methyl-3,6,6-triphenyl-1,2,4,5-tetraoxane) showed superior activity against chloroquine resistant RKL-9 strain (IC50 = 3.906 ± 0.006 μg/mL) in in contrast to all other derivatives of the set studied. In order to elucidate the vital drug interaction with falcipain-2 (FP-2), docking studies of potent ligands were performed.



Similar content being viewed by others
References
WHO World Malaria Report 2015, ISBN 978 92 4 156515 8; www.who.int (accessed 09.01.2017).
D. A. Casteel, in: Burger’s Medicinal Chemistry and Drug Discovery, Vol. 5, Wiley Interscience Publication, New York (1997), pp 3 – 75.
M. K. Kumawat and D. Chetia, Bangladesh J. Pharmacol., 10, 917 – 923 (2015).
M. K. Kumawat, U. P. Singh, B. Singh, et al., Arab. J. Chem., 9, S643 – S647 (2016).
X. Su and L. H. Miller, Sci. China Life Sci., 58(11), 1175 – 1179 (2015).
L. L. Franco, M. V. Almeida, L. F. R. Silva, et al., Chem. Biol. Drug Design,79, 790 – 797 (2012).
P. M. O’Neill, R. K. Amewu, G. L. Nixon, et al., Angew. Chem. Int. Ed., 49, 5693 – 5697 (2010).
P. M. O’Neill, G. L. Ellis, R. Amewu, et al., J. Med. Chem., 51(7), 2170 – 2177 (2008).
J. L. Vennerstrom, Y. Dong, S. L. Andersen, et al., J. Med. Chem., 43, 2753–2758 (2000).
J. L. Vennerstrom, J. Hong-Ning Fu, W. Y. Ellis, et al., J. Med. Chem., 35, 3023 – 3027 (1992).
K. Zmitek, S. Stavber, M. Zupan, et al., Bioorg. Med. Chem. Lett., 14, 7790 – 7795 (2006).
N. Kumar, S. I. Khan, H. Atheaya, et al., Euro. J. Med. Chem., 46, 2816 – 2827 (2011).
N. Kumar, S. I. Khan, Beena Negi, et al., Bioorg. Med. Chem., 17, 5632 – 5638 (2009).
N. Kumar, S. I. Khan, M. Sharma, et al., Bioorg. Med. Chem., 19, 1675 – 1677 (2009).
D. Opsenica, D. E. Kyle, W. K. Milhous, et al., J. Serb. Chem. Soc., 68(4 – 5), 291 – 302 (2003).
J. Coates, in: Encyclopedia of Analytical Chemistry, R. A. Meyers, (Ed.), John Wiley & Sons Ltd., Chichester (2000), pp 10815 – 10837.
D. J. Pasto, C. R. Johnson, and M. J. Miller, Experiments and Techniques in Organic Chemistry, Prentice Hall, New Jersey (1992).
R. M. Silverstein and F. X. Webster, Spectrometric Identification of Organic Compounds, John Wiley and Sons Ltd., New York (1963).
D. W. Mathieson, Interpretation of Organic Spectra, Academic Press, New York (1965).
A. O. Terent’ev, D. A. Borisov, and I. A. Yaremenko, Chem. Heterocycl. Compd., 48(1), 55 – 58 (2012).
I. Opsenica, D. Opsenica, K. S. Smith, et al., J. Med. Chem., 51(7), 2261 – 2266 (2008).
G. L. Ellis, R. Amewu, C. Hall, et al., Bioorg. Med. Chem. Lett., 18, 1720 – 1724 (2008).
S. K. Puri and N. Singh, Exp. Parasitol.,94, 8 (2000).
B. S. Kalra, S. Chawla, P. Gupta, et al., Indian J. Pharmacol., 38(1), 5 – 11 (2006).
K. H. Rieckmann, L. J. Sax, G. H. Campbell, et al., Lancet, 1, 22 – 23 (1978).
W. Trager and J. B. Jensen, Science, 193, 673 – 675 (1976).
C. Lambros and J. P. Vanderberg, J. Parasitol., 65, 418 – 420 (1979).
Z. Bikadi and E. Hazai, J. Cheminf., 1, 15 (1997).
T. A. Halgren, J. Comput. Chem., 17(5 – 6), 490 – 519 (1998).
G. M. Morris and D. S. Goodsell, J. Comput. Chem., 19(14), 1639 – 1662 (1998).
F. J. Solis and R. J. B. Wets, Math. Oper. Res., 6(1), 19 – 30 (1981).
Acknowledgements
The authors are thankful to Dr.C.R.Pillai, Emeritus Scientist, and Dr. Anup Anvikar, Director, National Institute of Malaria Research (Indian Council of Medical Research), New Delhi for providing antimalarial screening facilities and training. The authors also are thankful to S.A.I.F., Punjab University, Chandigarh, India for providing spectroscopic data.
Conflict of Interest
The authors declare that they have no any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumawat, M.K., Singh, U.P. & Chetia, D. Design and Discovery of 3,6-Substituted 1,2,4,5-Tetraoxanes as New Class of Falcipain-2 Inhibitors for Antimalarial Action. Pharm Chem J 53, 822–830 (2019). https://doi.org/10.1007/s11094-019-02085-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02085-x