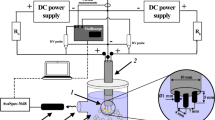
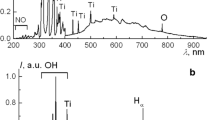
Abstract
Pulsed underwater direct current discharge is considered as a tool for a one-step process for ferrite synthesis and organic dye removal. The formation of cobalt, nickel and titanium ferrites during the discharge firing process was confirmed by methods of dynamic light scattering and X-ray phase analysis. The transformation of dye molecules (fluorescein, methylene green) during the combined action of plasma and ferrites was detected by UV absorption spectroscopy. The contributions of the separate action of plasma and ferrites to the process of dye removal from the solution were investigated. It was found that the synthesized structures have a high sorption capacity. It was found that fluorescein can be used as an indicator for the presence of nickel ferrites.










Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rezaei F, Vanraes P, Nikiforov A, Morent R, De Geyter N (2019) Applications of plasma-liquid systems: a review. Materials 12:2751
Kim S, Kim CH (2021) Applications of plasma-activated liquid in the medical field. Biomedicines 9:1700
Zhao Y, Bhavya ML, Patange A, Sun DW, Tiwari BK (2023) Plasma-activated liquids for mitigating biofilms on food and food contact surfaces. Compr Rev Food Sci Food Saf 22:1654–1685
Belmonte T, Nominé AV, Noël C, Gries T, Nominé A, Milichko V, Awaji MY (2023) Submerged discharges in liquids for nanoobject synthesis: expectations and capabilities. Plasma Chem Plasma Process 1–56. https://doi.org/10.1007/s11090-023-10349-4
Toriyabe Y, Watanabe S, Yatsu S, Shibayama T, Mizuno T (2007) Controlled formation of metallic nanoballs during plasma electrolysis. Appl Phys Lett 91:041501
GyoáKoo I, SeokáLee M, HeeáShim J, HwanáAhn J, MooáLee (2005) Platinum nanoparticles prepared by a plasma-chemical reduction method. J Mater Chem 15:4125–4128
Hieda J, Saito N, Takai O (2008) Exotic shapes of gold nanoparticles synthesized using plasma in aqueous solution. J Vacuum Sc Technol A: Vacuum Surf Films 26:854–856
Bratescu MA, Cho SP, Takai O, Saito N (2011) Size-controlled gold nanoparticles synthesized in solution plasma. J Phys Chem C 115:24569–24576
Kim SM, Lee YJ, Kim JW, Lee SY (2014) Facile synthesis of Pt–Pd bimetallic nanoparticles by plasma discharge in liquid and their electrocatalytic activity toward methanol oxidation in alkaline media. Thin Solid Films 572:260–265
Shuaib U, Hussain T, Ahmad R, Zakaullah M, Mubarik FE, Muntaha ST, Ashraf S (2020) Plasma-liquid synthesis of silver nanoparticles and their antibacterial and antifungal applications. Mater Res Express 7:035015
Saito G, Hosokai S, Tsubota M, Akiyama T (2011) Synthesis of copper/copper oxide nanoparticles by solution plasma. J Appl Phys 110:023302
Kelgenbaeva Z, Omurzak E, Takebe S, Sulaimankulova S, Abdullaeva Z, Iwamoto C, Mashimo T (2014) Synthesis of pure iron nanoparticles at liquid–liquid interface using pulsed plasma. J Nanoparticle Res 16:2603
Hattori Y, Mukasa S, Toyota H, Inoue T, Nomura S (2011) Synthesis of zinc and zinc oxide nanoparticles from zinc electrode using plasma in liquid. Mater Lett 65:188–190
Farajimotlagh M, Poursalehi R, Aliofkhazraei M (2017) Synthesis mechanisms, optical and structural properties of η-Al2O3 based nanoparticles prepared by DC arc discharge in environmentally friendly liquids. Ceram Int 43:7717–7723
Goli M, Haratizadeh H, Abrishami ME (2014) Growth of flower-like copper oxide nanostructures by glow discharge in water. Ceram Int 40:16071–16075
Li CJ, Cao X, Li WH, Zhang BW, **ao LQ (2019) Co-synthesis of CuO-ZnO nanoflowers by low voltage liquid plasma discharge with brass electrode. J Alloys Compnds 773:762–769
Kim TH, Jeong SJ, Lim HR, Cho HB, Lee CG, Choa YH (2018) Bulk-direct synthesis of TiO2 nanoparticles by plasma-assisted electrolysis with enhanced photocatalytic performance. J Electrochem Soc 165:E64
Chen L, Mashimo T, Okudera H, Iwamoto C, Omurzak E (2014) Synthesis of WO3·H2O nanoparticles by pulsed plasma in liquid. RSC Adv 4:28673–28677
Wang B, Yu J, Lu Q, **ao Z, Ma X, Feng Y (2022) Preparation of Mn3O4 microspheres via glow discharge electrolysis plasma as a high-capacitance supercapacitor electrode material. J Alloys Compnds 926:166775
Khlyustova AV, Sirotkin NA, Krayev AS, Titov VA, Agafonov AV (2019) Synthesis of MoO3 by glow discharge in contact with water. Plasma Sci Technol 21:025505
Khlyustova A, Sirotkin N, Kraev A, Titov V, Agafonov A (2020) Plasma–liquid synthesis of MoO x and WO 3 as potential photocatalysts. Dalt Trans 49:6270–6279
Shabani M, Saebnoori E, Hassanzadeh-Tabrizi SA, Bakhsheshi-Rad HR (2021) Novel synthesis of nickel ferrite magnetic nanoparticles by an in-liquid plasma. J Mater Sci Mater Electron 32:10424–10442
Smirnova KV, Shutov DA, Ivanov AN, Ivanova PA, Manukyan AS, Rybkin VV (2024) Cobalt ferrites: formation from nitrate solutions under the action of DC discharge. Plasma Chem Plasma Process 44:257–268
Khlyustova AV, Shipko MN, Stepovich MA, Agafonov AV, Sirotkin NA, Savchenko ES (2023) Composition and magnetic properties of composites based on ultrafine NiFe2O4 particles produced under conditions of low-temperature underwater plasma. Bull Russ Acad Sci Phys 87:1549–1551
Rakshit R, Khatun E, Pal M, Talukdar S, Mandal D, Saha P, Mandal K (2017) Influence of functional group of dye on the adsorption behaviour of CoFe2O4 nano-hollow spheres. New J Chem 41:9095–9102
Bakhshayesh S, Dehghani H (2014) Nickel and cobalt ferrites nanoparticles: synthesis, study of magnetic properties and their use as magnetic adsorbent for removing lead (II) ion. J Iran Chem Soc 11:769–780
Springer V, Barreiros L, Avena M, Segundo MA (2018) Nickel ferrite nanoparticles for removal of polar pharmaceuticals from water samples with multi-purpose features. Adsorption 24:431–441
Hameed TA, Azab AA, Ibrahim RS, Rady KE (2022) Optimization, structural, optical and magnetic properties of TiO2/CoFe2O4 nanocomposites. Ceram Int 48:20418–20425
Sun Q, Wu S, Li K, Han B, Chen Y, Pang B, Dong L (2020) The favourable synergistic operation of photocatalysis and catalytic oxygen reduction reaction by a novel heterogeneous CoFe2O4-TiO2 nanocomposite. Appl Surf Sci 516:146142
de Cristo Borges GC, Verdi IR, Fidelis MZ, Junior HEZ, Lenzi GG, de Souza ÉCF, Brackmann R (2023) Magnetic heterostructures of NiFe2O4 and TiO2: Pechini synthesis, characterization and photocatalytic performance in arsenite oxidation. J Water Process Eng 56:104352
Duque JS, Madrigal BM, Riascos H, Avila YP (2019) Colloidal metal oxide nanoparticles prepared by laser ablation technique and their antibacterial test. Colloids Interfaces 3:25
Tabassi NR, Ghasemiyan R, Brandkam MR, Hosseinpour T, Abkenar SK, Nesaz FR, Salehzadeh A (2022) Green synthesis of TiFe2O4@Ag nanocomposite using Spirulina platensis; characterization of their anticancer activity and evaluation of their effect on the expression of Bax, p53, and Bcl-2 genes in AGS cell line. J Cluster Sci 33:1601–1611
Akimoto SI, Katsura T, Yoshida M (1957) Magnetic properties of TiFe2O4-Fe3O4 system and their change with oxidation. J Geomag Geoelec 9:165–178
Mudarra Navarro AM, Gil Rebaza AV, Salcedo Rodriguez KL, Melo Quintero JJ, RodrÍguez Torres CE, Weissmann M, Errico LA (2019) Structural, electronic, and magnetic properties and hyperfine interactions at the Fe sites of the spinel TiFe2O4. Ab Initio, XANES, and Mossbauer study. J Phys Chem C 123:21694–21703
Adewuyi A, Oderinde RA (2022) Titanium ferrite–doped zeolitic imidazolate framework: an efficient catalyst for converting underutilized Khaya senegalensis seed oil to biodiesel. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03589-5
Khlyustova AV, Sirotkin NA, Kraev AS, Titov VA, Agafonov AV (2021) Synthesis and characterization of titanium oxide nanoparticles by plasma in contact with liquid. Plasma Chem Plasma Process 41:643–657
Sirotkin N, Khlyustova A (2022) The oxide nanostructures formation mechanisms in underwater plasma in terms of electrochemistry. Plasma Chem Plasma Process 42:1003–1013
Fried D, Reck GP, Kushida T, Rothe EW (1991) Time-resolved emission spectra following the 193-nm photoablation of CuO, BaO2, Y2O3, and YBa2Cu3O7 – x in vacuum and oxygen. J Appl Phys 70:2337–2342
Kelm K, Mader W (2005) Synthesis and structural analysis of ϵ-Fe2O3. Z Anorg All Chem 631:2383–2389
Khlyustova AV, Shipko MN, Sirotkin NA, Agafonov AV, Stepovich MA (2022) Using low-temperature plasma in contact with liquid to obtain nanostructured iron oxides. Bull Russ Acad Sci Phys 86:509–515
Sirotkin N, Korolev V (2023) Synthesis and characteristics of graphene–graphene oxide material obtained by an underwater impulse direct current discharge. Plasma Chem Plasma Process 43:1077–1092
Saeed R, Nadeem SMS (2016) The kinetics of electron transfer reaction of methylene green and titanium trichloride in different solvents. Russ J Phys Chem A 90:1143–1150
Yazdani O, Irandoust M, Ghasemi JB, Hooshmand S (2012) Thermodynamic study of the dimerization equilibrium of methylene blue, methylene green and thiazole orange at various surfactant concentrations and different ionic strengths and in mixed solvents by spectral titration and chemometric analysis. Dyes Pigm 92:1031–1041
Liu Z, Yin S, Zhang R, Zhu W, Fu P (2021) High-efficiency synthesis of carbon-bridged dimers via bioinspired green dimerization involving aldehydes. ACS Sust Chem Eng 10:655–661
Siejak P, Fra̧ckowiak D (2005) Spectral properties of fluorescein molecules in water with the addition of a colloidal suspension of silver. J Phys Chem B 109:14382–14386
Pirillo S, Cornaglia L, Ferreira ML, Rueda EH (2008) Removal of Fluorescein using different iron oxides as adsorbents: effect of pH. Spectrochim Acta Mol Biomol Spectrosc 71:636–643
Khlyustova AV, Maksimov AI, Panova DS (2013) Effect of electric discharges and oxidizing agents on aqueous solutions of a mixture of two organic dyes. Surf Eng Appl Electrochem 49:272–277. https://doi.org/10.3103/S1068375513040078
Acknowledgements
Authors would like to thank Dr. D. Smirnova for conducting XRD analysis, Dr. M. Yurov for performing SEM analysis, Dr. Yu. Fadeeva for conducting the FTIR analysis, and Dr. N. Kochkina for peforming DLS measurements at the center of joint use of scientific equipment (the Upper Volga Regional Center for Physical-Chemical Research, Russia).
Funding
This work was performed in the frame of the Government Assignment of the Ministry of Education and Science of Russia (no. 0092-2019-0004).
Author information
Authors and Affiliations
Contributions
A.K. wrote the main manuscript text. N.S. conducted electrical and spectral measurements. A.K. carried out chemical analysis and interpretation of structural analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khlyustova, A., Sirotkin, N. One-Stage Method for Removing Dyes under the Action of Underwater Plasma and Ferrites of Cobalt, Nickel, and Titanium. Plasma Chem Plasma Process (2024). https://doi.org/10.1007/s11090-024-10471-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11090-024-10471-x