Abstract
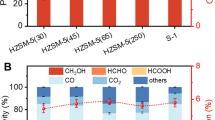
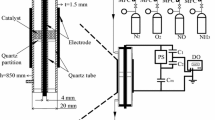
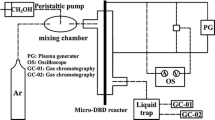
In this paper, the conversion of methane to methanol on CuO/Al2O3 and Mo–CuO/Al2O3 catalysts in a plasma reactor was tested. A comparison between catalytic and plasma-catalytic systems had been made in tested temperature range of 50–300°C. Experimental results showed that plasma-catalytic system demonstrated a much better methane conversion than catalytic system in tested temperature range and Mo–CuO/Al2O3 revealed a higher catalytic activity than CuO/Al2O3 for methanol synthesis. Furthermore, an Arrhenius plot was made in order to deduce the mechanism of plasma activation, which revealed that the presence of plasma decreased the activation energy for both catalysts. In the case of Mo-CuO/Al2O3 catalyst, the enhanced activity for methanol synthesis was assumed due to the oxygen vacancies on Mo–CuO/Al2O3 catalyst, which can utilize plasma-induced species to improve the catalytic efficiency.









Similar content being viewed by others
References
Michael M, Thomas S, Herrmann WA (2002) Angew Chem Int Ed 41:1745
Shiota Y, Yoshizawa K (2000) J Am Chem Soc 122:12317
Maack M, Friis-Jensen H, Sckerl S, Larsen JH, Chorkendorff I (2003) Top Catal 22:151
Schulz H (1999) Appl Catal A 186:3
Aasberg-Petersen K, Bak Hansen JH, Christensen TS, Dybkjaer I, Christensen PS, Stub Nielsen C, Winter Madsen SEL, Rostrup-Nielsen JR (2001) Appl Catal A 221:379
Taylor SH, Hargreaves JSJ, Hutchings GJ, Joyner RW, Lembacher CW (1998) Catal Today 42:217
Michalkiewicz B (2004) Appl Catal A 277:147
Omata K, Fukuoka N, Fujimoto K (1994) Ind Eng Chem Res 33:784
Nozaki T, Hattori A, Okazaki K (2004) Catal Today 98:607
Aghamir FM, Matin NS, Jalili AH, Esfarayeni MH, Khodagholi MA, Ahmadi R (2004) Plasma Sources Sci Technol 13:707
Cooray V, Rahman M (2005) J Electrostat 63:977
Subrahmanyam C, Renken A, Kiwi-Minsker L (2007) Chem Eng J 134:78
Yamamoto T (1997) J Electrostat 42:227
Morent R, Leys C, Dewulf J, Neirynck D, Van Durme J, Van Langenhove H (2007) J Adv Oxid Technol 10:127
Nagao I, Nishida M, Yukimura K, Kambara S, Maruyama T (2002) Vacuum 65:481
Eliasson B, Kogelschatz U (1991) Plasma Sci IEEE Trans 19:309
Kunhardt EE (2000) Plasma Sci IEEE Trans 28:189
Kim S-S, Kwon B, Kim J (2007) Catal Commun 8:2204
Pietruszka B, Heintze M (2004) Catal Today 90:151
Da Costa P, Marques R, Da Costa S (2008) Appl Catal B 84:214
Wang C-H, Lin S-S, Liou S-B, Weng H-S (2002) Chemosphere 49:389
Kado S, Sekine Y, Nozaki T, Okazaki K (2004) Catal Today 89:47
Demidyuk V, Whitehead J (2007) Plasma Chem Plasma Process 27:85
Strunk J, Kähler K, **a X, Comotti M, Schüth F, Reinecke T, Muhler M (2009) Appl Catal A 359:121
Widmann D, Leppelt R, Behm RJ (2007) J Catal 251:437
Acknowledgments
We appreciated the financial support to this study by the National Natural Science Foundation of China (No. 90610005, 20836008 and U0633003). The Project of science and technology department of Zhejiang province of China (2007C13061) and MOST project of China (No. 2007AA06Z339; No. 2008BAC32B06; No. 2007AA06A409) are also appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, L., Zhang, Xw., Chen, L. et al. Direct Oxidation of Methane to Methanol Over Cu-Based Catalyst in an AC Dielectric Barrier Discharge. Plasma Chem Plasma Process 31, 67–77 (2011). https://doi.org/10.1007/s11090-010-9272-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-010-9272-1