Abstract
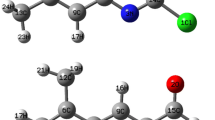
Computational studies based on Density Functional Theory (DFT) and time-dependent DFT (TD-DFT) were used to determine molecular and thermodynamic parameters, optoelectronic and electronic properties, as well as chemical descriptors, UV–vis spectra and nonlinear optical response of the chromophores of 2-Styrylquinoline (M01) and 2-(3-nitrostyryl)quinoline (M02). We evaluated the impact of the substitution of the hydrogen atom in M01 by the nitro group (NO2) to obtain the molecule M02. The results show very good optoelectronic properties with respect to very high electric fields of the order of 1.43 × 109 Vm−1 for M01 up to 5.26 × 109 Vm−1 for M02. The energy gap decreases from 3.94 eV for M01 to 3.56 eV for M02; which suggest that they are semiconductors. In addition, these two molecules exhibit ionization potential (IP) and electron affinity (EA) quite close to those of Alq3 and could therefore be good candidates for the manufacture of Organic Light-Emitting Diodes (OLED). The chemical descriptors were assessed and allowed us to discuss the stability and reactivity of our molecules. Thus, the addition of the nitro group to the virgin 2-Styrylquinoline decreases the reactivity and stability of the 2-Styrylquinoline. The analysis of the IR and Raman vibrational spectra shows several peaks between 100 cm−1 and 3200 cm−1 of which the most intense for the molecule M01 are attributed to the stretching mode of the C–H bond in the phenyl ring and the C=C double bond in the quinoline ring. For the M02 molecule, stronger peaks were assigned to the stretching mode of the O–N and N–C bonds. The analysis of the UV–vis spectra showed that the maximum absorption wavelength of M01 is 334.80 nm and that of M02 is 375.37 nm; both located in the ultraviolet. The high values of first (\({\beta }_{T}\)) and second (\(\overline{\gamma }\)) hyperpolarizability showed that both molecules have very good nonlinear optical properties and thus can be used for second and third harmonic generation.






Similar content being viewed by others
Data availability
The authors declare that: (i) The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. (ii) Some data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Andersson, M.R., et al.: Electroluminescence from substituted poly(thiophenes): from blue to near-infrared. Macromolecules 28, 7525–7529 (1995). https://doi.org/10.1021/ma00126a033
Assatse, Y.T., et al.: Theoretical studies of nanostructures modeled by the binding of Uracil derivatives to functionalized (5,5) carbon nanotubes. Chem. Phys. Lett. 136602, 731 (2019). https://doi.org/10.1016/j.cplett.2019.136602
Ayalew, M.E.: DFT studies on molecular structure, thermodynamics parameters, HOMO-LUMO and spectral analysis of pharmaceuticals compound quinoline (Benzo[b]Pyridine). J. Biophys. Chem. 3, 13 (2022). https://doi.org/10.4236/jbpc.2022.133003
Becke, A.D.: Densityfunctional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993). https://doi.org/10.1063/1.464913
Bouba, M.O., et al.: DFT investigation of Percyanation effect of coronene molecule Comparative study with their Perhalogenated counterparts. Polym. Bull. 79, 9663–9684 (2022). https://doi.org/10.1007/s00289-021-03967-5
Bredas, J.-L.: Mind the gap. Royal Soc. Chem. (materials Horzons). 1, 17–19 (2014). https://doi.org/10.1039/c3mh00098b
Budyka, M.F.: Design principles and action of molecular logic gates. Russian Chem. Bull. Int. Edition. 63, 1656–1665 (2014). https://doi.org/10.1007/s11172-014-0651-2
Budyka, M.F., et al.: Molecular logic gates based on 2 styrylquinoline derivatives. Russian Chem. Bull. Int. Edition. 57, 2586–2591 (2008a). https://doi.org/10.1007/s11172-008-0372-5
Budyka, M.F., et al.: Photochemical properties of amino and nitro derivatives of 2- and 4-styrylquinolines and their hydrochlorides. High Energy Chem. 42, 220–226 (2008b). https://doi.org/10.1134/S0018143908030065
Budyka, M.F., et al.: Photoisomerization of 2-styrylquinoline in neutral and protonated forms. High Energy Chem. 42, 446–453 (2008c). https://doi.org/10.1134/S0018143908060052
Budyka, M.F., et al.: Reconfigurable molecular logic gate operating in polymer film. J. Mater. Chem. 19, 7721–7724 (2009). https://doi.org/10.1039/B908562A
Cassidy, C., et al.: Nonlinear optical properties of urea. Opt. Commun. (1979). https://doi.org/10.1016/0030-4018(79)90027-0
Chai, J.D., Head-Gordon, M.: Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008). https://doi.org/10.1039/B810189B
Dennington R., Keith J. and Millam J. Gauss view, version 6. - [s.l.] : Semichem Inc, Shawnee Mission, 2016.
Derkowska-Zielinska, B., et al.: Optical properties of polymethacrylate with styrylquinoline side chains. Proc. of SPIE. 9652, 965216–965221 (2015). https://doi.org/10.1117/12.2194840
Dixon, D.A., Matsuzawa, N.: Density functional study of the structures and nonlinear optical properties of urea. J. Phys. Chem. 98, 3967–3971 (1994). https://doi.org/10.1021/j100066a011
Donga, S., et al.: Theoretical studies on charge transport character and optional properties of Alq3 and its difluorinated derivatives. Synth. Met. 159, 385–390 (2009). https://doi.org/10.1016/j.synthmet.2008.10.012
Ejuh, G.W., et al.: Computational determination of the Electronic and Nonlinear Optical properties of the molecules 2-(4-aminophenyl) Quinoline, 4-(4-aminophenyl) Quinoline, Anthracene, Anthraquinone and Phenanthrene. Mat. Let. 178, 221–226 (2016). https://doi.org/10.1016/j.matlet.2016.04.097
Ejuh, G.W., et al.: Electronic structure, physico-chemical, linear and nonlinear optical properties analysis of coronene,6B-, 6N-, 3B3N- substituted C24H12 using RHF, B3LYP and wB97XD methods. Opt. Quant. Electron. 49, 382 (2017). https://doi.org/10.1007/s11082-017-1221-2
Ejuh, G.W., et al.: Theoretical study on the electronic, optoelectronic, linear and non linear optical properties and UV–Vis Spectrum of Coronene and Coronene substituted with Chlorine. SN Appl. Sci. 1247, 2 (2020). https://doi.org/10.1007/s42452-020-3028-1
Espinosa, R., et al.: Synthesis and evaluation of the in vitro and in vivo antitrypanosomal activityof 2-styrylquinolines. Heliyon 7, e07024 (2021). https://doi.org/10.1016/j.heliyon.2021.e07024
Fankam, F.J.B., et al.: Study of electronic structure, optoelectronics, linear and nonlinear optical properties and chemical descriptors of dibromodinitrofluorescein isomers in gas phase and solvent media using Ab Initio and DFT methods. Chin. J. Phys. 66, 461–473 (2020). https://doi.org/10.1016/j.cjph.2020.05.015
Foster, M.E., Wong, B.M.: Nonempirically tuned range-separated DFT accurately predicts both fundamental and excitation gaps in DNA and RNA nucleobases. J. Chem. Theory Comput. 8, 2682–2687 (2012). https://doi.org/10.1021/ct300420f
Fouejio, D., et al.: DFT studies of the structural, chemical descriptors and nonlinear optical properties of the drug dihydroartemisinin functionalized on C60 fullerene. Comput. Theor. Chem. 1202, 113298 (2021). https://doi.org/10.1016/j.comptc.2021.113298
Frisch, M., et al.: Gaussian 09, Revision D01. - [s.l.] : Gaussian, Inc., Wallingford CT, 2013
Galiazzo, G., Gennari, G., Bortolus, P.: 3-styrylquinoline conformers: a photophysical and photochemical study. J. Photochem. 23, 149–161 (1983). https://doi.org/10.1016/0047-2670(83)80057-4
Gennari, G., Galiazzo, G., Bortolus, P.: Solvent dependence of the excited state reactivity of 1-styrylisoquinoline. J. Photochem. Photobiol. a: Chem. 43, 293–302 (1988). https://doi.org/10.1016/1010-6030(88)80026-1
Gennari, G., Bortolus, P., Galiazzo, G.: Conformational equilibrium in trans-aza-aryl-ethylenes: n-styrylquinolines and n-styrylisoquinolines. J. Mol. Struct. 249, 189–202 (1991). https://doi.org/10.1016/0022-2860(91)85067-D
Gennari, G., Caurzo, G., Galiarzo, G.: Cis-Trans Photoisomerization of β-Styrylnaphthalene and 3-Styrylquinoline. J. Phys. Chem. 81, 1551–1554 (1997). https://doi.org/10.1021/j100531a006
Gubler, U., Bosshard, C.: Optical third-harmonic generation of fused silica in gas atmosphere: absolute value of the third-order nonlinear optical susceptibility χ(3). Phys. Rev. B 61, 10702–10710 (2000). https://doi.org/10.1103/PhysRevB.61.10702
Guichaoua, D., et al.: UV irradiation induce NLO modulation in photochromic styrylquinoline-based polymers: computational and experimental studies. Org. Electron. 66, 175–182 (2019). https://doi.org/10.1016/j.orgel.2018.12.022
Gündüza, B., Kurban, M.: Photonic spectroscopic properties and electronic structure of PTCDI-C8 organic nanostructure. Vib. Spectrosc. 96, 46–51 (2018). https://doi.org/10.1016/j.vibspec.2018.02.008
Gunter P. Nonlinear Optical Effects and Materials [Book]. - [s.l.] : Springer Series in Optical Sciences, 2000.
Jamróz, M.H.: Vibrational energy distribution analysis (VEDA): Scopes and limitations. Spectrochimica Acta Part A 114, 220–230 (2013). https://doi.org/10.1016/j.saa.2013.05.096
Jamróz M.H. Vibrational Energy Distribution Analysis VEDA 4 Warsaw. - 2004.
Jenekhe, S.A., et al.: Large third-order optical nonlinearities in organic polymer superlattices. Appl. Phys. Lett. 57(2), 126–128 (1990)
Jensen, L., Duijnen, P.T.V.: The first hyperpolarizability of p-nitroaniline in 1,4-dioxane: a quantum mechanical/molecular mechanics study. J. Chem. Phys. 123, 074307 (2005). https://doi.org/10.1063/1.1999633
Kajzara, F., et al.: Second- and third-order non-linear optical properties of multilayered structures and composites of C60 with electron donors. Syn. Metals 117, 189–193 (2001). https://doi.org/10.1016/S0379-6779(00)00498-7
Kamsi, R.Y., et al.: Study of the molecular structure, electronic and chemical properties of Rubescin D molecule. Chin. J. Phys. 63, 104–121 (2020). https://doi.org/10.1016/j.cjph.2019.10.016
Kharchenko, O., et al.: Design, synthesis, and photochemistry of styrylquinoline-containing polymers. Mol. Crystals Liquid Crystals. 640, 71–77 (2016). https://doi.org/10.1080/15421406.2016.1255516
Kumru, M., Küçük, V., Kocademir, M.: Determination of structural and vibrational properties of 6-quinolinecarboxaldehyde using FT-IR FT-Raman and Dispersive-Raman experimental techniques and theoretical HF and DFT (B3LYP) methods. Spectrochimica Acta Part a: Mol. and Biomol. Spectros. 96, 242–251 (2012). https://doi.org/10.1016/j.saa.2012.05.001
Kurtz S. K., Jerphagnon J. and Choy M. M. Nonlinear Dielectric Susceptibilities (Landolt-Bornstein: Numerical Data and Functional Relationships in Science and Technology - New Series (ed. K. H. K. Hellwege), IIII11, chap. 6), Berlin : Springer, (1979).
Kuzmina, L.G., et al.: Molecular Structures and Crystal Packings of 2 Styrylquinoline and Its Derivatives. Crystallogr. Rep. 56, 611–621 (2011). https://doi.org/10.1134/S1063774511040110
Larkin, P.: Infrared and Raman Spectroscopy: Principles and Spectral Interpretation. Elsevier, USA (2011)
Leupacher, W., Penzkofer, A.: Third-order nonlinear susceptibilities of dye solutions determined by third-harmonic generation. Appl. Phys. B 36, 25–31 (1985). https://doi.org/10.1007/BF00698033
Li, Y.: Organic Optoelectronic Materials. Springer, Cham (2015)
Li, V.M., Gavrishova, T.N., Budyka, M.F.: Microwave-assisted solvent-free synthesis of 2-styrylquinolines in the presence of zinc chloride. Russian J. Organic Chem. 48, 826–831 (2012). https://doi.org/10.1134/S1070428012060139
Magri, A., et al.: Charge carrier mobility and electronic properties of Al(Op)3: impact of excimer formation. Beilstein J. Nanotechnol. 6, 1107–1115 (2015). https://doi.org/10.3762/bjnano.6.112
Marenich, A.V., Cramer, C.J., Truhlar, D.G.: Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009). https://doi.org/10.1021/jp810292n
Mveme, C.D.D., et al.: Density functional theory study of optoelectronic, nonlinear optical, piezoelectric and thermodynamic properties of poly (3,4-ethylenedioxythiophene), poly(3,4-ethylenedioxyselenophene) and their derivatives. Opt. Quantum Electr. (2020). https://doi.org/10.1007/s11082-020-02492-5
Noudem, P., et al.: Hartree-Fock and DFT studies of the optoelectronic, thermodynamic, structural and nonlinear optical properties of photochromic polymers containing styrylquinoline fragments. Mater. Chem. Phys. 281, 125883 (2022a). https://doi.org/10.1016/j.matchemphys.2022.125883
Noudem, P., et al.: Impact of do** on the optoelectronic, electronic and nonlinear optical properties and on the reactivity of photochromic polymers containing styrylquinoline fragments: Hartree-Fock and DFT study. Heliyon 8, e11491 (2022b). https://doi.org/10.1016/j.heliyon.2022.e11491
Oboyle, N.M., Tenderholt, A.L., Langner, K.M.: Software news and updates cclib: a library for package-independent computational chemistry algorithms. J. Comput. Chem. 29, 839–845 (2008). https://doi.org/10.1002/jcc.20823
Ostroverkhova, O.: Handbook of organic materials for optical and (opto)electronic devices: Properties and applications. Woodhead Publishing Limited, Oxford, Cambridge, Philadelphia, New Delhi (2013)
Ouazzani, H.E., et al.: Second- and third-order nonlinearities of novel push-pull azobenzene polymers. J. Phys. Chem. B 115, 1944–1949 (2011). https://doi.org/10.1021/jp109936t
Parr, R., Pearson, R.: Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983). https://doi.org/10.1021/ja00364a005
Parr, R.G., Szentpály, L.V., Liu, S.: Electrophilicity index. J. Am. Chem. Soc. 121, 1922 (1999)
Pearson, R.G.: Chemical hardness and density functional theory. J. Chem. Sci. 117, 369–377 (2005). https://doi.org/10.1007/BF02708340
Popczyk, A., et al.: Selected organometallic compounds for third order nonlinear optical application. Nanomaterials (2019). https://doi.org/10.3390/nano9020254
Radziszewski, J.G., et al.: Infrared absorption spectroscopy of the phenyl radical. J. Am. Chem. Soc. 118, 7400–7401 (1996). https://doi.org/10.1021/ja960617+
Reis, H., et al.: Calculation of macroscopic linear and nonlinear optical susceptibilities for the naphthalene, anthracene and meta-nitroaniline crystals. Chem. Phys. 261, 359–371 (2000). https://doi.org/10.1016/S0301-0104(00)00305-0
Rodrigues, R.F.N., et al.: Solid state characterization and theoretical study of non-linear optical properties of a Fluoro-N-Acylhydrazide derivative. PLoS ONE (2017). https://doi.org/10.1371/journal.pone.0175859
Samyn, C., Verbiest, T., Persoons, A.: Second-order non-linear optical polymers. Macromol. Rapid Commun. 21, 1–15 (2000)
Santos, L.F., Gozzi, G.: Electrical properties of polymer light-emitting devices [Book]. - [s.l.] : Conducting Polymers - Faris Yilmaz (2016)
Schmidt, A., Anderson, M.L., Armstrong, N.R.: Electronic states of vapor deposited electron and hole transport agents and luminescent materials for light-emitting diodes. J. Appl. Phys. 78, 5619–5625 (1995). https://doi.org/10.1063/1.359685
Shakerzadeh, E., Kazemimoghadam, F., Chigo, A.E.: How does lithiation affect electro-optical features of corannulene (c20h10) and quadrannulene (C16H8) buckybowls? J. Electr. Mater. 47, 2348–2358 (2018). https://doi.org/10.1007/s11664-018-6069-0
Shim, S.C., Kim, D.W., Kim, M.S.: Studies on the conformational equilibrium of trans-2-styrylquinoline. J. Photochem. Photobiol. a: Chem. 56, 227–238 (1991). https://doi.org/10.1016/1010-6030(91)80023-B
Socrates, G.: Infrared & Raman characteristic group frequencies : tables & charts, 3rd Ed. [Book]. - [s.l.] : Lavoisier, 2004
Ulahannan, R.T., et al.: Vibrational spectroscopic studies and molecular docking study of 2-[(E)-2-phenylethenyl]quinoline-5-carboxylic acid. Spectrochim Acta A Mol Biomol Spectrosc. 150, 190–199 (2015). https://doi.org/10.1016/j.saa.2015.04.104
Valle, G., Busetti, V., Galiazzo, G.: Crystal structure of E-2-styrylquinoline(STQ) C17H13N Zeitschrift für Kristallographie. Crystal. Mater 177, 315–318 (1986). https://doi.org/10.1524/zkri.1986.177.3-4.315
Wong, B.M., Hsieh, T.H.: Optoelectronic and excitonic properties of oligoacenes: substantial Improvements from range-separated time-dependent density functional theory. J. Chem. Theory Comput. 6, 3704–3712 (2010). https://doi.org/10.1021/ct100529s
Yang, W., Parr, R.G.: Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc. Nati. Acad. Sci. 82, 6723–6726 (1985). https://doi.org/10.1073/pnas.82.20.6723
Zawadzka, A., et al.: Photophysical properties of Alq3 thin films. Opt. Mater. 36, 91–97 (2014). https://doi.org/10.1016/j.optmat.2013.05.001
Acknowledgements
We are thankful to the Council of Scientific and Industrial Research (CSIR), India, for its financial support through Emeritus Professor scheme (grant no. 21(0582)/03/EMR-II) to Prof. A.N. Singh of the Physics Department, Banaras Hindu University, India, which enabled him to purchase the Gaussian Software. Our gratitude also goes to late Emeritus Prof. A.N. Singh for donating this software to Prof Geh Wilson Ejuh. We also thank the Center for Education and Industrial Technical Training (CEFTI) for enabling us used their computing facilities.
Funding
The authors declare that this research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. DF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. CDDM: Conceptualization, Investigation. FTN: Methodology. SSZ: Formal analysis, Supervision, Validation, Writing—original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noudem, P., Fouejio, D., Mveme, C.D.D. et al. Theoretical investigations of the electronic structure, spectroscopic (IR, Raman and UV–Vis), optoelectronic, thermodynamic and nonlinear optical properties of chromophores of 2-styrylquinoline and 2-(3-nitrostyryl)quinoline. Opt Quant Electron 55, 1240 (2023). https://doi.org/10.1007/s11082-023-05495-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-05495-0