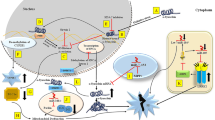
Abstract
Epigenetic modulations play a major role in gene expression and thus are responsible for various physiological changes including age-associated neurological disorders. Neurodegenerative diseases such as Alzheimer’s (AD), Parkinson’s (PD), Huntington’s disease (HD), although symptomatically different, may share common underlying mechanisms. Most neurodegenerative diseases are associated with increased oxidative stress, aggregation of certain proteins, mitochondrial dysfunction, inactivation/dysregulation of protein degradation machinery, DNA damage and cell excitotoxicity. Epigenetic modulations has been reported to play a significant role in onset and progression of neurodegenerative diseases by regulating these processes. Previous studies have highlighted the marked antioxidant and neuroprotective abilities of polyphenols such as curcumin, by increased activity of detoxification systems like superoxide dismutase (SOD), catalase or glutathione peroxidase. The role of curcumin as an epigenetic modulator in neurological disorders and neuroinflammation apart from other chronic diseases have also been reported by a few groups. Nonetheless, the evidences for the role of curcumin mediated epigenetic modulation in its neuroprotective ability are still limited. This review summarizes the current knowledge of the role of mitochondrial dysfunction, epigenetic modulations and mitoepigenetics in age-associated neurological disorders such as PD, AD, HD, Amyotrophic Lateral Sclerosis (ALS), and Multiple Sclerosis (MS), and describes the neuroprotective effects of curcumin in the treatment and/or prevention of these neurodegenerative diseases by regulation of the epigenetic machinery.


Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Kim-Ha J, Kim YJ (2016) Age-related epigenetic regulation in the brain and its role in neuronal diseases. BMB Rep 49(12):671–680. https://doi.org/10.5483/bmbrep.2016.49.12.184
Ayeni EA, Aldossary AM, Ayejoto DA, Gbadegesin LA, Alshehri AA, Alfassam HA, Afewerky HK, Almughem FA, Bello SM, Tawfik EA (2022) Neurodegenerative diseases: implications of environmental and climatic influences on neurotransmitters and neuronal hormones activities. Int J Environ Res Public Health 19(19):12495. https://doi.org/10.3390/ijerph191912495
Maresova P, Javanmardi E, Barakovic S, Barakovic Husic J, Tomsone S, Krejcar O, Kuca K (2019) Consequences of chronic diseases and other limitations associated with old age—a sco** review. BMC Public Health 19(1):1431. https://doi.org/10.1186/s12889-019-7762-5
Atanasov AG, Zotchev SB, Dirsch VM et al (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 20:200–216. https://doi.org/10.1038/s41573-020-00114-z
Arts IC, Hollman PC (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81(1):317S-325S. https://doi.org/10.1093/ajcn/81.1.317S
Arias-Sánchez RA, Torner L, Fenton Navarro B (2023) Polyphenols and neurodegenerative diseases: potential effects and mechanisms of neuroprotection. Molecules (Basel, Switzerland) 28(14):5415. https://doi.org/10.3390/molecules28145415
Casadesus G, Shukitt-Hale B, Stellwagen HM, Zhu X, Lee HG, Smith MA, Joseph JA (2004) Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr Neurosci 7(5–6):309–316. https://doi.org/10.1080/10284150400020482
Karuppagounder SS, Madathil SK, Pandey M, Haobam R, Rajamma U, Mohanakumar KP (2013) Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 236:136–148. https://doi.org/10.1016/j.neuroscience.2013.01.032
Zhang Y, Yu W, Zhang L, Wang M, Chang W (2022) The interaction of polyphenols and the gut microbiota in neurodegenerative diseases. Nutrients 14(24):5373. https://doi.org/10.3390/nu14245373
Yan L, Guo MS, Zhang Y et al (2022) Dietary plant polyphenols as the potential drugs in neurodegenerative diseases: current evidence, advances, and opportunities. Oxid Med Cell Longev 2022:5288698. https://doi.org/10.1155/2022/5288698
Tiffon C (2018) The impact of nutrition and environmental epigenetics on human health and disease. Int J Mol Sci 19(11):3425. https://doi.org/10.3390/ijms19113425
Korosi SJ, Layé AS et al (2017) Food for thought: how nutrition impacts cognition and emotion. npj Sci Food 1:7. https://doi.org/10.1038/s41538-017-0008-y
Gómez-Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9(7):568–578. https://doi.org/10.1038/nrn2421
Ferguson LR (2015) Nutritional modulation of gene expression: might this be of benefit to individuals with Crohn’s disease? Front Immunol 6:467. https://doi.org/10.3389/fimmu.2015.00467
Hodges RE, Minich DM (2015) Modulation of metabolic detoxification pathways using foods and food-derived components: a scientific review with clinical application. J Nutr Metab 2015:760689. https://doi.org/10.1155/2015/760689
Dawson DR 3rd, Branch-Mays G, Gonzalez OA, Ebersole JL (2014) Dietary modulation of the inflammatory cascade. Periodontol 2000 64(1):161–197. https://doi.org/10.1111/j.1600-0757.2012.00458.x
Pan MH, Lai CS, Dushenkov S, Ho CT (2009) Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem 57(11):4467–4477. https://doi.org/10.1021/jf900612n
Vasconcelos AR, Dos Santos NB, Scavone C, Munhoz CD (2019) Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front Pharmacol 10:33. https://doi.org/10.3389/fphar.2019.00033
Virmani A, Pinto L, Binienda Z, Ali S (2013) Food, nutrigenomics, and neurodegeneration neuroprotection by what you eat! Mol Neurobiol 48(2):353–362. https://doi.org/10.1007/s12035-013-8498-3
Pacholko AG, Wotton CA, Bekar LK (2019) Poor diet, stress, and inactivity converge to form a “perfect storm” that drives Alzheimer’s disease pathogenesis. Neurodegener Dis 19(2):60–77. https://doi.org/10.1159/000503451
Wu Q, Gao ZJ, Yu X, Wang P (2022) Dietary regulation in health and disease. Signal Transduct Target Ther 7(1):252. https://doi.org/10.1038/s41392-022-01104-w
Chaiwangyen W (2021) The impact of dietary compounds in functional foods on MicroRNAs expression. IntechOpen. https://doi.org/10.5772/intechopen.96746
Rodrigo R, Libuy M, Feliu F, Hasson D (2014) Polyphenols in disease: from diet to supplements. Curr Pharm Biotechnol 15(4):304–317. https://doi.org/10.2174/138920101504140825113815
Hewlings SJ, Kalman DS (2017) Curcumin: a review of its effects on human health. Foods 6(10):92. https://doi.org/10.3390/foods6100092
Amalraj A, Pius A, Gopi S, Gopi S (2016) Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. J Tradit Complement Med 7(2):205–233. https://doi.org/10.1016/j.jtcme.2016.05.005
Susana RM, Joyce T, Chaverri JP (2018) Utility of curcumin for the treatment of diabetes mellitus: evidence from preclinical and clinical studies. J Nutr Intermed Metab 14:29–41. https://doi.org/10.1016/j.jnim.2018.05.001
Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K, Zielińska D, Salehi B, Setzer WN, Dosoky NS, Taheri Y, El Beyrouthy M, Martorell M, Ostrander EA, Suleria HAR, Cho WC, Maroyi A, Martins N (2020) Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical biotechnological and medicinal applications. Front Pharmacol 11:01021. https://doi.org/10.3389/fphar.2020.01021
Shal B, Ding W, Ali H, Kim YS, Khan S (2018) Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer’s disease. Front Pharmacol 9:548. https://doi.org/10.3389/fphar.2018.00548
Rahman MM, Rahaman MS, Islam MR, Rahman F, Mithi FM, Alqahtani T, Almikhlafi MA, Alghamdi SQ, Alruwaili AS, Hossain MS, Ahmed M, Das R, Emran TB, Uddin MS (2021) Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules 27(1):233. https://doi.org/10.3390/molecules27010233
Aggarwal BB, Sung B (2009) Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 30(2):85–94. https://doi.org/10.1016/j.tips.2008.11.002
Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R (2013) Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 11(4):338–378. https://doi.org/10.2174/1570159X11311040002
Yadav SK, Sah AK, Jha RK, Sah P, Shah DK (2013) Turmeric (curcumin) remedies gastroprotective action. Pharmacogn Rev 7(13):42–46. https://doi.org/10.4103/0973-7847.112843
Giordano A, Tommonaro G (2019) Curcumin and cancer. Nutrients 11(10):2376. https://doi.org/10.3390/nu11102376
Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15(1):195–218. https://doi.org/10.1208/s12248-012-9432-8
Pourhanifeh MH, Darvish M, Tabatabaeian J et al (2020) Therapeutic role of curcumin and its novel formulations in gynecological cancers. J Ovarian Res 13(1):130. https://doi.org/10.1186/s13048-020-00731-7
Epstein J, Sanderson IR, Macdonald TT (2010) Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 103(11):1545–1557. https://doi.org/10.1017/S0007114509993667
Ma W, Xu D, Zhao L, Yuan M, Cui YL, Li Y (2023) Therapeutic role of curcumin in adult neurogenesis for management of psychiatric and neurological disorders: a scientometric study to an in-depth review. Crit Rev Food Sci Nutr 63(28):9379–9391. https://doi.org/10.1080/10408398.2022.2067827
Abidi A, Gupta S, Agarwal M, Bhalla HL, Saluja M (2014) Evaluation of efficacy of curcumin as an add-on therapy in patients of bronchial asthma. J Clin Diagn Res 8(8):HC19–HC24. https://doi.org/10.7860/JCDR/2014/9273.4705
Wu S, **ao D (2016) Effect of curcumin on nasal symptoms and airflow in patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol 117(6):697-702.e1. https://doi.org/10.1016/j.anai.2016.09.427
Birdane L, Cingi C, Muluk NB, San T, Burukoglu D (2016) Evaluation of the efficacy of curcumin in experimentally induced acute sinusitis in rats. Ear Nose Throat J 95(12):21–27 (PMID: 27929603)
Cole GM, Teter B, Frautschy SA (2007) Neuroprotective effects of curcumin. Adv Exp Med Biol 595:197–212. https://doi.org/10.1007/978-0-387-46401-5_8
Seddon N, D’Cunha NM, Mellor DD, McKune AJ, Georgousopoulou EN, Panagiotakos DB et al (2019) Effects of curcumin on cognitive function—a systematic review of randomized controlled trials. Explor Res Hypothesis Med. 4(1):1–11. https://doi.org/10.14218/ERHM.2018.00024
Marton LT, Pescinini-E-Salzedas LM, Camargo MEC et al (2021) The effects of curcumin on diabetes mellitus: a systematic review. Front Endocrinol 12:669448. https://doi.org/10.3389/fendo.2021.669448
Ramaholimihaso T, Bouazzaoui F, Kaladjian A (2020) Curcumin in depression: potential mechanisms of action and current evidence—a narrative review. Front Psychiatry 11:572533. https://doi.org/10.3389/fpsyt.2020.572533
Nebrisi EE (2021) Neuroprotective activities of curcumin in Parkinson’s disease: a review of the literature. Int J Mol Sci 22(20):11248. https://doi.org/10.3390/ijms222011248
Maiti P, Dunbar GL (2018) Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int J Mol Sci 19(6):1637. https://doi.org/10.3390/ijms19061637
Karthikeyan A, Senthil N, Min T (2020) Nanocurcumin: a promising candidate for therapeutic applications. Front Pharmacol 11:487. https://doi.org/10.3389/fphar.2020.00487
Mukherjee S, Mishra AK, Peer GDG et al (2021) The interplay of the unfolded protein response in neurodegenerative diseases: a therapeutic role of curcumin. Front Aging Neurosci 13:767493. https://doi.org/10.3389/fnagi.2021.767493
Ghosh S, Banerjee S, Sil PC (2015) The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol 83:111–124. https://doi.org/10.1016/j.fct.2015.05.022
Benameur T, Giacomucci G, Panaro MA et al (2021) New promising therapeutic avenues of curcumin in brain diseases. Molecules 27(1):236. https://doi.org/10.3390/molecules27010236
Sokolik VV, Shulga SM (2015) Effect of curcumin liposomal form on angiotensin converting activity, cytokines and cognitive characteristics of the rats with Alzheimer’s disease model. Biotechnologia Acta 8(6):48–56. https://doi.org/10.15407/biotech8.06.048
Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB (2011) Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr 6(2):93–108. https://doi.org/10.1007/s12263-011-0222-1
Fabianowska-Majewska K, Kaufman-Szymczyk A, Szymanska-Kolba A, Jakubik J, Majewski G, Lubecka K (2021) Curcumin from turmeric rhizome: a potential modulator of DNA methylation machinery in breast cancer inhibition. Nutrients 13(2):332. https://doi.org/10.3390/nu13020332
Hassan FU, Rehman MS, Khan MS et al (2019) Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Front Genet 10:514. https://doi.org/10.3389/fgene.2019.00514
Teiten MH, Dicato M, Diederich M (2013) Curcumin as a regulator of epigenetic events. Mol Nutr Food Res 57(9):1619–1629. https://doi.org/10.1002/mnfr.201300201
Boyanapalli SS, Kong AT (2015) “Curcumin, the King of Spices”: epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr Pharmacol Rep 1(2):129–139. https://doi.org/10.1007/s40495-015-0018-x
Dadhania VP, Trivedi PP, Vikram A, Tripathi DN (2016) Nutraceuticals against neurodegeneration: a mechanistic insight. Curr Neuropharmacol 6:627–640. https://doi.org/10.2174/1570159x14666160104142223
Behl T, Kaur G, Sehgal A et al (2021) Elucidating the multi-targeted role of nutraceuticals: a complementary therapy to starve neurodegenerative diseases. Int J Mol Sci 22(8):4045. https://doi.org/10.3390/ijms22084045
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 8(21):2003–2014. https://doi.org/10.3969/j.issn.1673-5374.2013.21.009
Jadiya P, Garbincius JF, Elrod JW (2021) Reappraisal of metabolic dysfunction in neurodegeneration: focus on mitochondrial function and calcium signaling. Acta Neuropathol Commun 9(1):124. https://doi.org/10.1186/s40478-021-01224-4
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1(1):a006189
Bamburg JR, Bloom GS (2009) Cytoskeletal pathologies of Alzheimer disease. Cell Motil Cytoskeleton 66(8):635–649. https://doi.org/10.1002/cm.20388
Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y (2020) Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener 15(1):40. https://doi.org/10.1186/s13024-020-00391-7
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
Zhao A, Li Y, Niu M et al (2020) SNPs in SNCA, MCCC1, DLG2, GBF1 and MBNL2 are associated with Parkinson’s disease in southern Chinese population. J Cell Mol Med 24(15):8744–8752. https://doi.org/10.1111/jcmm.15508
Langmyhr M, Henriksen SP, Cappelletti C et al (2021) Allele-specific expression of Parkinson’s disease susceptibility genes in human brain. Sci Rep 11:504. https://doi.org/10.1038/s41598-020-79990-9
Yang J, Ke S, Qiao Z et al (2021) Interactions between glycogen synthase kinase-3β gene polymorphisms, negative life events, and susceptibility to major depressive disorder in a Chinese population. Front Psychiatry 11:503477. https://doi.org/10.3389/fpsyt.2020.503477
Kwok JB, Loy CT, Hamilton G et al (2008) Glycogen synthase kinase-3beta and tau genes interact in Alzheimer’s disease. Ann Neurol 64(4):446–454. https://doi.org/10.1002/ana.21476
Chen J, Long Z, Li Y, Luo M, Luo S, He G (2019) Alteration of the Wnt/GSK3β/βcatenin signalling pathway by rapamycin ameliorates pathology in an Alzheimer’s disease model. Int J Mol Med 44(1):313–323. https://doi.org/10.3892/ijmm.2019.4198
Pedersen CC, Lange J, Førland MGG, Macleod AD, Alves G, Maple-Grødem J (2021) A systematic review of associations between common SNCA variants and clinical heterogeneity in Parkinson’s disease. NPJ Parkinson’s disease 7(1):54. https://doi.org/10.1038/s41531-021-00196-5
DaRocha-Souto B, Coma M, Pérez-Nievas BG et al (2012) Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol Dis 45(1):425–437. https://doi.org/10.1016/j.nbd.2011.09.002
Simunovic F, Yi M, Wang Y et al (2009) Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson’s disease pathology. Brain 132(Pt 7):1795–1809. https://doi.org/10.1093/brain/awn323
Jia E, Zhou Y, Liu Z et al (2020) Transcriptomic profiling of circular RNA in different brain regions of Parkinson’s disease in a mouse model. Int J Mol Sci 21(8):3006. https://doi.org/10.3390/ijms21083006
Wu GS (2004) The functional interactions between the MAPK and p53 signaling pathways. Cancer Biol Ther 3(2):156–161. https://doi.org/10.4161/cbt.3.2.614
Montarolo F, Perga S, Martire S et al (2016) Altered NR4A subfamily gene expression level in peripheral blood of Parkinson’s and Alzheimer’s disease patients. Neurotox Res 30(3):338–344. https://doi.org/10.1007/s12640-016-9626-4
Mythri RB, Raghunath NR, Narwade SC, Pandareesh MDR, Sabitha KR, Aiyaz M, Srinivas Bharath MM (2017) Manganese-and 1-methyl-4-phenylpyridinium-induced neurotoxicity display differences in morphological, electrophysiological and genome-wide alterations: implications for idiopathic Parkinson’s disease. J Neurochem 143(3):334–358. https://doi.org/10.1111/jnc.14147
Glaab E, Schneider R (2015) Comparative pathway and network analysis of brain transcriptome changes during adult aging and in Parkinson’s disease. Neurobiol Dis 74:1–13. https://doi.org/10.1016/j.nbd.2014.11.002
Bagyinszky E, Giau VV, An SA (2020) Transcriptomics in Alzheimer’s disease: aspects and challenges. Int J Mol Sci 21(10):3517. https://doi.org/10.3390/ijms21103517
Wang M, Roussos P, McKenzie A et al (2016) Integrative network analysis of nineteen brain regions identifies molecular signatures and networks underlying selective regional vulnerability to Alzheimer’s disease. Genome Med 8:104. https://doi.org/10.1186/s13073-016-0355-3
Belenguer P, Duarte JM, Schuck PF, Ferreira GC (2019) Mitochondria and the brain: bioenergetics and beyond. Neurotox Res 36:219–238. https://doi.org/10.1007/s12640-019-00061-7
Xu S, Zhang X, Liu C, Liu Q, Chai H, Luo Y, Li S (2021) Role of mitochondria in neurodegenerative diseases: from an epigenetic perspective. Front Cell Dev Biol 27(9):688789. https://doi.org/10.3389/fcell.2021.688789
Abramov AY, Angelova PR (2019) Mitochondrial dysfunction and energy deprivation in the mechanism of neurodegeneration. Turk J Biochem 44(6):723–729. https://doi.org/10.1515/tjb-2019-0255
Beckhauser TF, Francis-Oliveira J, De Pasquale R (2016) Reactive oxygen species: physiological and physiopathological effects on synaptic plasticity. J Exp Neurosci 10:23–48. https://doi.org/10.4137/JEN.S39887
Olufunmilayo EO, Gerke-Duncan MB, Damian-Holsinger RM (2023) Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 12(2):517. https://doi.org/10.3390/antiox12020517
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57:1105–1121. https://doi.org/10.3233/JAD-161088
Manczak M, Calkins MJ, Reddy PH (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet 20:2495–2509. https://doi.org/10.1093/hmg/ddr139
Rice AC, Keeney PM, Algarzae NK, Ladd AC, Thomas RR, Bennett JPJ (2014) Mitochondrial DNA copy numbers in pyramidal neurons are decreased and mitochondrial biogenesis transcriptome signaling is disrupted in Alzheimer’s disease hippocampi. J Alzheimers Dis 40:319–330. https://doi.org/10.3233/JAD-131715
Monzio Compagnoni G, Di Fonzo A, Corti S, Comi GP, Bresolin N, Masliah E (2020) The role of mitochondria in neurodegenerative diseases: the lesson from Alzheimer’s disease and Parkinson’s disease. Mol Neurobiol 57:2959–2980. https://doi.org/10.1007/s12035-020-01926-1
Hattori N, Kitada T, Matsumine H, Asakawa S, Yamamura Y, Yoshino H, Kobayashi T, Yokochi M, Wang M, Yoritaka A, Kondo T, Kuzuhara S, Nakamura S, Shimizu N, Mizuno Y (1998) Molecular genetic analysis of a novel Parkin gene in Japanese families with autosomal recessive juvenile parkinsonism: evidence for variable homozygous deletions in the Parkin gene in affected individuals. Ann Neurol 44:935–941. https://doi.org/10.1002/ana.410440612
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B et al (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–1160. https://doi.org/10.1126/science.1096284
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787. https://doi.org/10.1038/nature05292
Orsini M, Oliveira AB, Nascimento OJ, Reis CHM, Leite MAA, de Souza JA, Pupe C, de Souza OG, Bastos VH, de Freitas MR (2015) Amyotrophic lateral sclerosis: new perpectives and update. Neurol Int. https://doi.org/10.4081/ni.2015.5885
Smith EF, Shaw PJ, De Vos KJ (2019) The role of mitochondria in amyotrophic lateral sclerosis. Neurosci Lett 710:132933. https://doi.org/10.1016/j.neulet.2017.06.052
Li S, Li XJ (2006) Multiple pathways contribute to the pathogenesis of Huntington disease. Mol Neurodegener 1:19. https://doi.org/10.1186/1750-1326-1-19
Li P, Marshall L, Oh G et al (2019) Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat Commun 10(1):2246. https://doi.org/10.1038/s41467-019-10101-7
Ghosh P, Saadat A (2021) Neurodegeneration and epigenetics: a review. Neurologia. https://doi.org/10.1016/j.nrl.2021.01.016
Yu Z, Wang T, Xu J et al (2015) Mutations in the glucocerebrosidase gene are responsible for Chinese patients with Parkinson’s disease. J Hum Genet 60(2):85–90. https://doi.org/10.1038/jhg.2014.110
Eryilmaz IE, Cecener G, Erer S et al (2017) Epigenetic approach to early-onset Parkinson’s disease: low methylation status of SNCA and PARK2 promoter regions. Neurol Res 39(11):965–972. https://doi.org/10.1080/01616412.2017.1368141
Blanch M, Mosquera JL, Ansoleaga B, Ferrer I, Barrachina M (2016) Altered mitochondrial DNA methylation pattern in Alzheimer disease-related pathology and in Parkinson disease. Am J Pathol 186(2):385–397. https://doi.org/10.1016/j.ajpath.2015.10.004
Zhou XW, Gustafsson JA, Tanila H et al (2008) Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol Dis 31(3):386–394. https://doi.org/10.1016/j.nbd.2008.05.013
Alberdi E, Sánchez-Gómez MV, Cavaliere F et al (2010) Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47(3):264–272. https://doi.org/10.1016/j.ceca.2009.12.010
Kuchibhotla KV, Wegmann S, Kopeikina KJ et al (2014) Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci U S A 111(1):510–514. https://doi.org/10.1073/pnas.1318807111
Nucifora FC Jr, Sasaki M, Peters MF et al (2001) Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291(5512):2423–2428. https://doi.org/10.1126/science.1056784
Lee J, Hwang YJ, Kim Y et al (2017) Remodeling of heterochromatin structure slows neuropathological progression and prolongs survival in an animal model of Huntington’s disease. Acta Neuropathol 134(5):729–748. https://doi.org/10.1007/s00401-017-1732-8
Migliore L, Coppedè F (2009) Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res 667(1–2):82–97. https://doi.org/10.1016/j.mrfmmm.2008.10.011
Matsumoto L, Takuma H, Tamaoka A et al (2010) CpG demethylation enhances alpha-synuclein expression and affects the pathogenesis of Parkinson’s disease. PLoS ONE 5(11):e15522. https://doi.org/10.1371/journal.pone.0015522
Skou LD, Johansen SK, Okarmus J, Meyer M (2024) Pathogenesis of DJ-1/PARK7-mediated Parkinson’s disease. Cells 13:296. https://doi.org/10.3390/cells13040296
Jowaed A, Schmitt I, Kaut O, Wüllner U (2010) Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients’ brains. J Neurosci 30(18):6355–6359. https://doi.org/10.1523/JNEUROSCI.6119-09.2010
Desplats P, Spencer B, Coffee E et al (2011) Alpha-synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J Biol Chem 286(11):9031–9037. https://doi.org/10.1074/jbc.C110.212589
Cai M, Tian J, Zhao GH, Luo W, Zhang BR (2011) Study of methylation levels of parkin gene promoter in Parkinson’s disease patients. Int J Neurosci 121(9):497–502. https://doi.org/10.3109/00207454.2011.580866
Goers J, Manning-Bog AB, McCormack AL et al (2003) Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry 42(28):8465–8471. https://doi.org/10.1021/bi0341152
Kim J, Inoue K, Ishii J et al (2007) A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317(5842):1220–1224. https://doi.org/10.1126/science.1140481
Vartiainen S, Pehkonen P, Lakso M, Nass R, Wong G (2006) Identification of gene expression changes in transgenic C. elegans overexpressing human alpha-synuclein. Neurobiol Dis 22(3):477–486. https://doi.org/10.1016/j.nbd.2005.12.021
Asikainen S, Rudgalvyte M, Heikkinen L et al (2010) Global microRNA expression profiling of Caenorhabditis elegans Parkinson’s disease models. J Mol Neurosci 41(1):210–218. https://doi.org/10.1007/s12031-009-9325-1
Breijyeh Z, Karaman R (2020) Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules 25(24):5789. https://doi.org/10.3390/molecules25245789
Guan JS, Haggarty SJ, Giacometti E et al (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459(7243):55–60. https://doi.org/10.1038/nature07925
Liu X, Jiao B, Shen L (2018) The epigenetics of Alzheimer’s disease: factors and therapeutic implications. Front Genet 9:579. https://doi.org/10.3389/fgene.2018.00579
Ding H, Dolan PJ, Johnson GV (2008) Histone deacetylase 6 interacts with the microtubule-associated protein tau. J Neurochem 106(5):2119–2130. https://doi.org/10.1111/j.1471-4159.2008.05564.x
Broide RS, Redwine JM, Aftahi N et al (2007) Distribution of histone deacetylases 1–11 in the rat brain. J Mol Neurosci 31(1):47–58. https://doi.org/10.1007/BF02686117
Wang Z, Xu P, Chen B et al (2018) Identifying circRNA-associated-ceRNA networks in the hippocampus of Aβ1–42-induced Alzheimer’s disease-like rats using microarray analysis. Aging (Albany NY). 10(4):775–788. https://doi.org/10.18632/aging.101427
Lee ST, Chu K, Jung KH et al (2012) miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol 72(2):269–277. https://doi.org/10.1002/ana.23588
Condliffe D, Wong A, Troakes C et al (2014) Cross-region reduction in 5-hydroxymethylcytosine in Alzheimer’s disease brain. Neurobiol Aging 35(8):1850–1854. https://doi.org/10.1016/j.neurobiolaging.2014.02.002
Landles C, Bates GP (2004) Huntingtin and the molecular pathogenesis of Huntington’s disease. Fourth in molecular medicine review series. EMBO Rep 5(10):958–963. https://doi.org/10.1038/sj.embor.7400250
Ryu H, Lee J, Hagerty SW et al (2006) ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington’s disease. Proc Natl Acad Sci U S A 103(50):19176–19181. https://doi.org/10.1073/pnas.0606373103
Zsindely N, Bodai L (2018) Histone methylation in Huntington’s disease: are bivalent promoters the critical targets? Neural Regen Res 13(7):1191–1192. https://doi.org/10.4103/1673-5374.235029
Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF, Pagani W, Lodin D, Orozco G, Chinea A (2015) A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int 16(6):171. https://doi.org/10.4103/2152-7806.169561
Floare ML, Allen SP (2020) Why TDP-43? Why not? Mechanisms of metabolic dysfunction in amyotrophic lateral sclerosis. Neurosci Insights. 15:2633105520957302. https://doi.org/10.1177/2633105520957302
Parrella E, Porrini V, Scambi I, Gennari MM, Gussago C, Bankole O, Benarese M, Mariotti R, Pizzi M (2022) Synergistic association of resveratrol and histone deacetylase inhibitors as treatment in amyotrophic lateral sclerosis. Front Pharmacol 13:1017364. https://doi.org/10.3389/fphar.2022.1017364
Klingl YE, Pakravan D, Van Den Bosch L (2021) Opportunities for histone deacetylase inhibition in amyotrophic lateral sclerosis. Br J Pharmacol 178(6):1353–1372. https://doi.org/10.1111/bph.15217
Rossaert E, Pollari E, Jaspers T, Van Helleputte L, Jarpe M, Van Damme P, De Bock K, Moisse M, Van Den Bosch L (2019) Restoration of histone acetylation ameliorates disease and metabolic abnormalities in a FUS mouse model. Acta Neuropathol Commun 7(1):107. https://doi.org/10.1186/s40478-019-0750-2
Kumar V, Kundu S, Singh A, Singh S (2022) Understanding the role of histone deacetylase and their inhibitors in neurodegenerative disorders: current targets and future perspective. Curr Neuropharmacol 20(1):158–178. https://doi.org/10.2174/1570159X19666210609160017
Calió ML, Henriques E, Siena A, Bertoncini CRA, Gil-Mohapel J, Rosenstock TR (2020) Mitochondrial dysfunction, neurogenesis, and epigenetics: putative implications for amyotrophic lateral sclerosis neurodegeneration and treatment. Front Neurosci 14:679. https://doi.org/10.3389/fnins.2020.00679
Rosen DR, Siddique T, Patterson D et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62. https://doi.org/10.1038/362059a0
Oates N, Pamphlett R (2007) An epigenetic analysis of SOD1 and VEGF in ALS. Amyotroph Lateral Scler 82:83–86. https://doi.org/10.1080/17482960601149160
Yang Y, Gozen O, Vidensky S, Robinson MB, Rothstein JD (2010) Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia 58:277–286. https://doi.org/10.1002/glia.20922
Tremolizzo L, Messina P, Conti E, Sala G, Cecchi M, Airoldi L et al (2014) Whole-blood global DNA methylation is increased in amyotrophic lateral sclerosis independently of age of onset. Amyotroph Lateral Scler Frontotemporal Degener 15:98–105. https://doi.org/10.3109/21678421.2013.851247
Martin LJ, Adams DA, Niedzwiecki MV, Wong M (2022) Aberrant DNA and RNA methylation occur in spinal cord and skeletal muscle of human SOD1 mouse models of ALS and in human ALS: targeting DNA methylation is therapeutic. Cells 11(21):3448. https://doi.org/10.3390/cells11213448
Bennett SA, Tanaz R, Cobos SN, Torrente MP (2019) Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease. Transl Res 204:19–30. https://doi.org/10.1016/j.trsl.2018.10.002
Ling SC (2018) Synaptic paths to neurodegeneration: the emerging role of TDP-43 and FUS in synaptic functions. Neural Plast 2018:8413496. https://doi.org/10.1155/2018/8413496
Di Pietro L, Baranzini M, Berardinelli MG et al (2017) Potential therapeutic targets for ALS: MIR206, MIR208b and MIR499 are modulated during disease progression in the skeletal muscle of patients. Sci Rep 7:9538. https://doi.org/10.1038/s41598-017-10161-z
Gacias M, Casaccia P (2014) Epigenetic mechanisms in multiple sclerosis. Rev Esp Escler Mult 6(29):25–35
Liggett T, Melnikov A, Tilwalli S, Yi Q, Chen H, Replogle C et al (2010) Methylation patterns of cell-free plasma DNA in relapsing-remitting multiple sclerosis. J Neurol Sci 290(1–2):16–21. https://doi.org/10.1016/j.jns.2009.12.018
Chomyk AM, Volsko C, Tripathi A et al (2017) DNA methylation in demyelinated multiple sclerosis hippocampus. Sci Rep 7:8696. https://doi.org/10.1038/s41598-017-08623-5
Kumagai C, Kalman B, Middleton FA, Vyshkina T, Massa PT (2012) Increased promoter methylation of the immune regulatory gene SHP-1 in leukocytes of multiple sclerosis subjects. J Neuroimmunol 246(1–2):51–57. https://doi.org/10.1016/j.jneuroim.2012.03.003
Küçükali Cİ, Kürtüncü M, Çoban A, Çebi M, Tüzün E (2015) Epigenetics of multiple sclerosis: an updated review. Neuromol Med 17:83–96. https://doi.org/10.1007/s12017-014-8298-6
Cipriano GL, Schepici G, Mazzon E, Anchesi I (2024) Multiple sclerosis: roles of miRNA, lcnRNA, and circRNA and their implications in cellular pathways. Int J Mol Sci 25(4):2255. https://doi.org/10.3390/ijms25042255
Thompson JW, Hu R, Huffaker TB, Ramstead AG, Ekiz HA, Bauer KM, Tang WW, Ghazaryan A, Round JL, Fu**ami RS et al (2023) MicroRNA-155 plays selective cell-intrinsic roles in brain-infiltrating immune cell populations during neuroinflammation. J Immunol 210:926–934. https://doi.org/10.4049/jimmunol.2200478
Lax NZ, Turnbull DM, Reeve AK (2011) Mitochondrial mutations: newly discovered players in neuronal degeneration. Neuroscientist 17(6):645–658. https://doi.org/10.1177/107385841138546
Coppedè F (2024) Mitochondrial DNA methylation and mitochondria-related epigenetics in neurodegeneration. Neural Regen Res 19(2):405–406. https://doi.org/10.4103/1673-5374.379045
Coppedè F, Stoccoro A (2019) Mitoepigenetics and neurodegenerative diseases. Front Endocrinol (Lausanne) 10:86. https://doi.org/10.3389/fendo.2019.00086
Calió ML, Henriques E, Siena A, Bertoncini CR, Gil-Mohapel J, Rosenstock TR (2020) Mitochondrial dysfunction, neurogenesis, and epigenetics: putative implications for amyotrophic lateral sclerosis neurodegeneration and treatment. Front Neurosci 14:679. https://doi.org/10.3389/.2020.00679
Blanch M, Mosquera JL, Ansoleaga B, Ferrer I, Barrachina M (2016) Altered mitochondrial DNA methylation pattern in Alzheimer disease-related pathology and in Parkinson disease. Am J Pathol 186:385–397. https://doi.org/10.1016/j.ajpath.2015.10.004
Xu Y, Xu L, Han M, Liu X, Li F, Zhou X, Wang Y, Bi J (2019) Altered mitochondrial DNA methylation and mitochondrial DNA copy number in an APP/PS1 transgenic mouse model of Alzheimer disease. Biochem Biophys Res Commun 520:41–46. https://doi.org/10.1016/j.bbrc.2019.09.094
Devall M, Soanes DM, Smith AR, Dempster EL, Smith RG, Burrage J, Iatrou A, Hannon E, Troakes C, Moore K, O’Neill P, Al-Sarraj S, Schalkwyk L, Mill J, Weedon M, Lunnon K (2022) Genome-wide characterization of mitochondrial DNA methylation in human brain. Front Endocrinol (Lausanne) 13:1059120. https://doi.org/10.3389/fendo.2022.1059120
Rivera J, Gangwani L, Kumar S (2023) Mitochondria localized microRNAs: An unexplored miRNA niche in Alzheimer’s disease and aging. Cells 12(5):742. https://doi.org/10.3390/cells1205074
Popa-Wagner A, Dumitrascu DI, Capitanescu B, Petcu EB, Surugiu R, Fang WH, Dumbrava DA (2020) Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen Res 15(3):394–400. https://doi.org/10.4103/1673-5374.26604
Gabbianelli R, Damiani E (2018) Epigenetics and neurodegeneration: role of early-life nutrition. J Nutr Biochem 57:1–13. https://doi.org/10.1016/j.jnutbio.2018.01.014
Sharma RP, Tun N, Grayson DR (2008) Depolarization induces downregulation of DNMT1 and DNMT3a in primary cortical cultures. Epigenetics 3:74–80. https://doi.org/10.4161/epi.3.2.6103
Olmedo-Díaz S, Estévez-Silva H, Orädd G, Af Bjerkén S, Marcellino D, Virel A (2017) An altered blood–brain barrier contrib to brain iron accumulation and neuroinflammation in the 6-OHDA rat model Parkinson’s disease. Neuroscience 362:141–151. https://doi.org/10.1016/j.neuroscience.2017.08.023
Schachtschneider KM, Liu Y, Rund LA, Madsen O, Johnson RW, Groenen MA et al (2016) Impact of neonatal iron deficiency on hippocampal DNA methylation and gene transcription in a porcine biomedical model of cognitive development. BMC Genomics 17(1):856. https://doi.org/10.1186/s12864-016-3216-y
Gerber H, Wu F, Dimitrov M, Garcia Osuna GM, Fraering PC (2017) (2017) Zinc and copper differentially modulate amyloid precursor protein processing by γ-secretase and amyloid-β peptide production. J Biol Chem 292(9):3751–3767. https://doi.org/10.1074/jbc.m116.754101
Wang C, Zhou C, Guo T, Huang P, Xu X, Zhang M (2022) Association between cigarette smoking and Parkinson’s disease: a neuroimaging study. Ther Adv Neurol Disord 15:17562864221092566. https://doi.org/10.1177/17562864221092566
Ren X, Chen JF (2020) Caffeine and Parkinson’s disease: multiple benefits and emerging mechanisms. Front Neurosci 14:602697. https://doi.org/10.3389/fnins.2020.60269
Yan R, Zhang J, Park HJ, Park ES, Oh S, Zheng H, Junn E, Voronkov M, Stock JB, Mouradian MM (2018) Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson’s disease and DLB. Proc Natl Acad Sci U S A 115(51):E12053–E12062. https://doi.org/10.1073/pnas.181336511
Sieurin J, Zhan Y, Pedersen NL, Wirdefeldt K (2021) Neuroticism, smoking, and the risk of Parkinson’s disease. J Parkinsons Dis 11(3):1325–1334. https://doi.org/10.3233/JPD-20252
Derkinderen P, Shannon KM, Brundin P (2014) Gut feelings about smoking and coffee in Parkinson’s disease. Mov Disord 29(8):976–979. https://doi.org/10.1002/mds.2588
Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9:124. https://doi.org/10.3389/fncel.2015.0012
Cannon JR, Greenamyre JT (2011) The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 124(2):225–250. https://doi.org/10.1093/toxsci/kfr239
Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, Coleman P, Lemere CA, Hof PR, van den Hove DL, Rutten BP (2015) The epigenetics of aging and neurodegeneration. Prog Neurobiol 131:21–64. https://doi.org/10.1016/j.pneurobio.2015.05.002
Shams H, Shao X, Santaniello A, Kirkish G, Harroud A, Ma Q, Isobe N, University of California San Francisco MS-EPIC Team, Schaefer CA, McCauley JL, Cree BAC, Didonna A, Baranzini SE, Patsopoulos NA, Hauser SL, Barcellos LF, Henry RG, Oksenberg JR (2023) Polygenic risk score association with multiple sclerosis susceptibility and phenotype in Europeans. Brain 146(2):645–656. https://doi.org/10.1093/brain/awac092
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5):270–278. https://doi.org/10.4161/oxim.2.5.9498
Vauzour D (2012) Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev 2012:914273. https://doi.org/10.1155/2012/914273
Mandel S, Weinreb O, Amit T, Youdim MB (2004) Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (–)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem 88(6):1555–1569. https://doi.org/10.1046/j.1471-4159.2003.02291.x
Das J, Ramani R, Suraju MO (2016) Polyphenol compounds and PKC signaling. Biochim Biophys Acta 10:2107–2121. https://doi.org/10.1016/j.bbagen.2016.06.022
Luccarini I, Grossi C, Rigacci S, Coppi E, Pugliese AM, Pantano D, La-Marca G, Ed-Dami T, Berti A, Stefani M, Casamenti F (2015) Oleuropein aglycone protects against pyroglutamylated-3 amyloid-ß toxicity: biochemical, epigenetic and functional correlates. Neurobiol Aging 36(2):648–663. https://doi.org/10.1016/J.neurobiolaging.2014.08.029
Ayissi VB, Ebrahimi A, Schluesenner H (2014) Epigenetic effects of natural polyphenols: a focus on SIRT1-mediated mechanisms. Mol Nutr Food Res 58(1):22–32. https://doi.org/10.1002/mnfr.201300195
Khan N, Mukhtar H (2013) Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochem Pharmacol 85(5):667–672. https://doi.org/10.1016/j.bcp.2012.09.027
Monroy A, Lithgow GJ, Alavez S (2013) Curcumin and neurodegenerative diseases. BioFactors 39(1):122–132. https://doi.org/10.1002/biof.1063
Zhang L, Fang Y, Cheng X, Lian Y, Zeng Z, Wu C, Zhu H, Xu H (2018) The potential protective effect of curcumin on amyloid-β-42 induced cytotoxicity in HT-22 cells. Biomed Res Int 15(2018):8134902. https://doi.org/10.1155/2018/8134902
He XJ, Uchida K, Megumi C, Tsuge N, Nakayama H (2015) Dietary curcumin supplementation attenuates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in C57BL mice. J Toxicol Pathol 28(4):197–206. https://doi.org/10.1293/tox.2015-0020
Jellinger KA (2010) Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med 14(3):457–487. https://doi.org/10.1111/j.1582-4934.2010.01010.x
Schmidt MF, Gan ZY, Komander D, Dewson G (2021) Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ 28(2):570–590. https://doi.org/10.1038/s41418-020-00706-7
Wang J, He Z (2009) NAD and axon degeneration: from the Wlds gene to neurochemistry. Cell Adh Migr 3(1):77–87. https://doi.org/10.4161/cam.3.1.7483
Krauss R, Bosanac T, Devraj R, Engber T, Hughes RO (2020) Axons matter: the promise of treating neurodegenerative disorders by targeting SARM1-mediated axonal degeneration. Trends Pharmacol Sci 41(4):281–293. https://doi.org/10.1016/j.tips.2020.01.006
Tegenge MA, Rajbhandari L, Shrestha S, Mithal A, Hosmane S, Venkatesan A (2014) Curcumin protects axons from degeneration in the setting of local neuroinflammation. Exp Neurol 253:102–110. https://doi.org/10.1016/j.expneurol.2013.12.016
Gao Y, Zhuang Z, Lu Y et al (2019) Curcumin mitigates neuro-inflammation by modulating microglia polarization through inhibiting TLR4 axis signaling pathway following experimental subarachnoid hemorrhage. Front Neurosci 13:1223. https://doi.org/10.3389/fnins.2019.01223
Mythri RB, Srinivas Bharath MM (2019) Curcumin for neurological and psychiatric disorders. Academic Press
Vasanthkumar T, Hanumanthappa M, Lakshminarayana R (2019) Curcumin and capsaicin modulates LPS induced expression of COX-2, IL-6 and TGF-β in human peripheral blood mononuclear cells. Cytotechnology 71(5):963–976. https://doi.org/10.1007/s10616-019-00338-x
Deng Y, Lu X, Wang L et al (2014) Curcumin inhibits the AKT/NF-κB signaling via CpG demethylation of the promoter and restoration of NEP in the N2a cell line. AAPS J 16(4):649–657. https://doi.org/10.1208/s12248-014-9605-8
Kang SK, Cha SH, Jeon HG (2006) Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev 15(2):165–174. https://doi.org/10.1089/scd.2006.15.165
Zhu X, Li Q, Chang R et al (2014) Curcumin alleviates neuropathic pain by inhibiting p300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS ONE 9(3):e91303. https://doi.org/10.1371/journal.pone.0091303
Funding
No funding.
Author information
Authors and Affiliations
Contributions
ST: Conceptualized the idea, prepared and finalized the manuscript, prepared the figures. B. Prepared the table and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Authors have no competing interest in any capacity.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tripathi, S., Bhawana Epigenetic Orchestration of Neurodegenerative Disorders: A Possible Target for Curcumin as a Therapeutic. Neurochem Res (2024). https://doi.org/10.1007/s11064-024-04167-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11064-024-04167-z