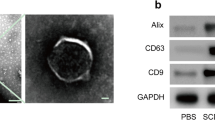
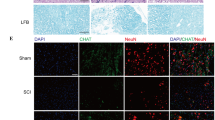
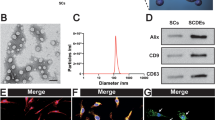
Abstract
Spinal cord injury (SCI) is a severe neurological condition that involves a lengthy pathological process. This process leads to the upregulation of chondroitin sulfate proteoglycans (CSPGs) by reactive glia, which impedes repair and regeneration in the spinal cord. The role of the CSPG-specific receptor protein tyrosine phosphatase-sigma (PTP-σ) in post-SCI remains largely unexplored. Exosomes have great potential in the diagnosis, prognosis, and treatment of SCI due to their ability to easily cross the blood‒brain barrier. Schwann cell-derived exosomes (SCDEs) promote functional recovery in mice post-SCI by decreasing CSPG deposition. However, the mechanism by which SCDEs decrease CSPGs after SCI remains unknown. Herein, we observed elevated levels of PTP-σ and increased CSPG deposition during glial scar formation after SCI in vivo. After SCDEs were injected into SCI mice, CSPG deposition decreased in scar tissue at the injury site, the expression of PTP-σ increased during axonal growth around the injury site, and motor function subsequently recovered. Additionally, we demonstrated that the use of both Rho/ROCK inhibitors and SCDEs inhibited the reparative effects of SCDEs on scar tissue after SCI. In conclusion, our study revealed that treatment with SCDEs targeting the Rho/ROCK signaling pathway reduced PTP-σ activation in the CSPG post-SCI, which inhibited scar tissue formation.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sterner RC, Sterner RM (2022) Immune response following traumatic spinal cord injury: pathophysiology and therapies. Front Immunol 13:1084101. https://doi.org/10.3389/fimmu.2022.1084101
Fan B et al (2018) Microenvironment Imbalance of spinal cord Injury. Cell Transpl 27:853–866. https://doi.org/10.1177/0963689718755778
Chen Y, Tang Y, Vogel LC, Devivo MJ (2013) Causes of spinal cord injury. Top Spinal Cord Inj Rehabil 19:1–8. https://doi.org/10.1310/sci1901-1
Ding WZ et al (2022) Spinal cord Injury: The Global incidence, prevalence, and disability from the global burden of Disease Study 2019. Spine 47:1532–1540. https://doi.org/10.1097/brs.0000000000004417
French DD et al (2007) Health care costs for patients with chronic spinal cord injury in the veterans health administration. J Spinal Cord Med 30:477–481. https://doi.org/10.1080/10790268.2007.11754581
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG (2014) Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 6:309–331. https://doi.org/10.2147/clep.S68889
Papa S et al (2021) Functionalized nanogel for treating activated astrocytes in spinal cord injury. J Control Release 330:218–228. https://doi.org/10.1016/j.jconrel.2020.12.006
Anjum A et al (2020) Spinal cord Injury: pathophysiology, Multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 21. https://doi.org/10.3390/ijms21207533
Orr MB, Gensel JC (2018) Spinal cord Injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15:541–553. https://doi.org/10.1007/s13311-018-0631-6
Dyck SM, Karimi-Abdolrezaee S (2015) Chondroitin sulfate proteoglycans: key modulators in the develo** and pathologic central nervous system. Exp Neurol 269:169–187. https://doi.org/10.1016/j.expneurol.2015.04.006
Milton AJ et al (2023) Recovery of Forearm and fine digit function after chronic spinal cord Injury by Simultaneous Blockade of Inhibitory Matrix Chondroitin Sulfate Proteoglycan Production and the receptor PTPσ. J Neurotrauma 40:2500–2521. https://doi.org/10.1089/neu.2023.0117
Shen Y et al (2009) PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592–596. https://doi.org/10.1126/science.1178310
Zuo S et al (2021) Identification of adhesion-associated DNA methylation patterns in the peripheral nervous system. Exp Ther Med 21:48. https://doi.org/10.3892/etm.2020.9479
Chen X et al (2021) Identification of anti-inflammatory vesicle-like nanoparticles in honey. J Extracell Vesicles 10:e12069. https://doi.org/10.1002/jev2.12069
Guo Y et al (2019) Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer 18. https://doi.org/10.1186/s12943-019-0995-1
Kim H et al (2019) Exosome-guided phenotypic switch of M1 to M2 macrophages for Cutaneous Wound Healing. Adv Sci (Weinheim Baden-Wurttemberg Germany) 6:1900513. https://doi.org/10.1002/advs.201900513
Jessen KR, Mirsky R, Lloyd AC (2015) Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol 7:a020487. https://doi.org/10.1101/cshperspect.a020487
Woodhoo A et al (2007) Schwann cell precursors: a favourable cell for myelin repair in the Central Nervous System. Brain 130:2175–2185. https://doi.org/10.1093/brain/awm125
Yu T, Yang LL, Zhou Y, Wu MF, Jiao JH (2024) Exosome-mediated repair of spinal cord injury: a promising therapeutic strategy. Stem Cell Res Ther 15:6. https://doi.org/10.1186/s13287-023-03614-y
Pan D et al (2021) Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J Neuroinflammation 18:172. https://doi.org/10.1186/s12974-021-02215-x
Wei Z et al (2019) Proteomics analysis of Schwann cell-derived exosomes: a novel therapeutic strategy for central nervous system injury. Mol Cell Biochem 457:51–59. https://doi.org/10.1007/s11010-019-03511-0
Bahram Sangani N, Gomes AR, Curfs LMG, Reutelingsperger CP (2021) The role of Extracellular vesicles during CNS development. Prog Neurobiol 205:102124. https://doi.org/10.1016/j.pneurobio.2021.102124
Pan D et al (2022) Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in mouse. Biotechnol Lett 44:129–142. https://doi.org/10.1007/s10529-021-03198-8
Ren J et al (2023) Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis 14:70. https://doi.org/10.1038/s41419-023-05607-4
Zhu B et al (2023) Schwann Cell-Derived exosomes and Methylprednisolone Composite Patch for spinal cord Injury Repair. ACS Nano 17:22928–22943. https://doi.org/10.1021/acsnano.3c08046
Ghosh M, Pearse DD (2023) Schwann Cell-Derived Exosomal vesicles: a promising therapy for the injured spinal cord. Int J Mol Sci 24. https://doi.org/10.3390/ijms242417317
Wong FC, Ye L, Demir IE, Kahlert C (2022) Schwann cell-derived exosomes: Janus-faced mediators of regeneration and disease. Glia 70:20–34. https://doi.org/10.1002/glia.24087
Watanabe K et al (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25:681–686. https://doi.org/10.1038/nbt1310
Watzlawick R et al (2014) Effect and reporting bias of RhoA/ROCK-blockade intervention on locomotor recovery after spinal cord injury: a systematic review and meta-analysis. JAMA Neurol 71:91–99. https://doi.org/10.1001/jamaneurol.2013.4684
Chan CC et al (2005) Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol 196:352–364. https://doi.org/10.1016/j.expneurol.2005.08.011
Zhang Y et al (2022) Rho kinase inhibitor Y27632 improves Recovery after spinal cord Injury by shifting astrocyte phenotype and morphology via the ROCK/NF-κB/C3 pathway. Neurochem Res 47:3733–3744. https://doi.org/10.1007/s11064-022-03756-0
Xu J et al (2023) Generation of neural organoids for spinal-cord regeneration via the direct reprogramming of human astrocytes. Nat Biomed Eng 7:253–269. https://doi.org/10.1038/s41551-022-00963-6
Harlow DE, Macklin WB (2014) Inhibitors of myelination: ECM changes, CSPGs and PTPs. Exp Neurol 251:39–46. https://doi.org/10.1016/j.expneurol.2013.10.017
Dyck SM et al (2015) Chondroitin sulfate proteoglycans negatively modulate spinal cord neural precursor cells by Signaling through LAR and RPTPσ and Modulation of the Rho/ROCK pathway. Stem Cells 33:2550–2563. https://doi.org/10.1002/stem.1979
Yamazaki K, Kawabori M, Seki T, Houkin K (2020) Clinical trials of stem cell treatment for spinal cord Injury. Int J Mol Sci 21. https://doi.org/10.3390/ijms21113994
Hashimoto S et al (2023) Microenvironmental modulation in tandem with human stem cell transplantation enhances functional recovery after chronic complete spinal cord injury. Biomaterials 295:122002. https://doi.org/10.1016/j.biomaterials.2023.122002
Zipser CM et al (2022) Cell-based and stem-cell-based treatments for spinal cord injury: evidence from clinical trials. Lancet Neurol 21:659–670. https://doi.org/10.1016/s1474-4422(21)00464-6
Cofano F et al (2019) Mesenchymal stem cells for spinal cord Injury: current options, limitations, and future of cell therapy. Int J Mol Sci 20. https://doi.org/10.3390/ijms20112698
Liu WZ, Ma ZJ, Li JR, Kang XW (2021) Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther 12:102. https://doi.org/10.1186/s13287-021-02153-8
40 Costăchescu B et al (2022) Novel strategies for spinal cord regeneration. Int J Mol Sci 23. https://doi.org/10.3390/ijms23094552
Chan WM, Mohammed Y, Lee I, Pearse DD (2013) Effect of gender on recovery after spinal cord injury. Transl Stroke Res 4:447–461. https://doi.org/10.1007/s12975-012-0249-7
Bianco A, Antonacci Y, Liguori M (2023) Sex and gender differences in neurodegenerative diseases: challenges for Therapeutic opportunities. Int J Mol Sci 24. https://doi.org/10.3390/ijms24076354
Ahmed RU et al (2023) Porcine spinal cord injury model for translational research across multiple functional systems. Exp Neurol 359:114267. https://doi.org/10.1016/j.expneurol.2022.114267
Dale K, Martí E (2017) Introduction to the special section: spinal cord a model to understand CNS development and regeneration. Dev Biol 432:1–2. https://doi.org/10.1016/j.ydbio.2017.10.005
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 82272470, 82072439]; the Tian** Health Key Discipline Special Project [grant number TJWJ2022XK011]; and the Outstanding Youth Foundation of Tian** Medical University General Hospital [grant number 22ZYYJQ01].
Author information
Authors and Affiliations
Contributions
GZN conceived and designed the research. HPM and SBZ supervised the project. HPM, SBZ and MFH conducted most experimental work. SBZ performed data analysis. HPM and SBZ wrote the manuscript. GZN reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tian** Medical University General Hospital (approval number: IRB2023-DW-88).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, S., Ma, H., Hou, M. et al. Schwann Cell-Derived Exosomes Induced Axon Growth after Spinal Cord Injury by Decreasing PTP-σ Activation on CSPGs via the Rho/ROCK Pathway. Neurochem Res 49, 2120–2130 (2024). https://doi.org/10.1007/s11064-024-04166-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-024-04166-0