Abstract
Vanilloids, including capsaicin and eugenol, are ligands of transient receptor potential channel vanilloid subfamily member 1 (TRPV1). Prolonged treatment with vanilloids triggered the desensitization of TRPV1, leading to analgesic or antinociceptive effects. Caenorhabditis elegans (C. elegans) is a model organism expressing vanilloid receptor orthologs (e.g., OSM-9 and OCR-2) that are associated with behavioral and physiological processes, including sensory transduction. We have shown that capsaicin and eugenol hamper the nocifensive response to noxious heat in C. elegans. The objective of this study was to perform proteomics to identify proteins and pathways responsible for the induced phenotype and to identify capsaicin and eugenol targets using a thermal proteome profiling (TPP) strategy. The results indicated hierarchical differences following Reactome Pathway enrichment analyses between capsaicin- and eugenol-treated nematodes. However, both treated groups were associated mainly with signal transduction pathways, energy generation, biosynthesis and structural processes. Wnt signaling, a specific signal transduction pathway, is involved following treatment with both molecules. Wnt signaling pathway is noticeably associated with pain. The TPP results show that capsaicin and eugenol target OCR-2 but not OSM-9. Further protein–protein interaction (PPI) analyses showed other targets associated with enzymatic catalysis and calcium ion binding activity. The resulting data help to better understand the broad-spectrum pharmacological activity of vanilloids.







Similar content being viewed by others
Data Availability
The data that support the findings from this study are available from the corresponding author upon reasonable request. Proteome discoverer 2.4 and Metascape datasets can be found online at Mendeley Data (https://doi.org/10.17632/4kv68ds8p5.1).
References
Glauser DA, Chen WC, Agin R et al (2011) Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics 188:91–103. https://doi.org/10.1534/genetics.111.127100
Goodman MB, Sengupta P (2019) How Caenorhabditis elegans senses mechanical stress, temperature, and other physical stimuli. Genetics 212:25–51. https://doi.org/10.1534/genetics.118.300241
Aoki I, Mori I (2015) Molecular biology of thermosensory transduction in C. elegans. Curr Opin Neurobiol 34:117–124. https://doi.org/10.1016/j.conb.2015.03.011
Schafer WR (2006) Proprioception: a channel for body sense in the worm. Curr Biol 16:R509–R511. https://doi.org/10.1016/j.cub.2006.06.012
Calahorro F, Izquierdo PG (2018) The presynaptic machinery at the synapse of C. elegans. Invertebr neurosci 18:4. https://doi.org/10.1007/s10158-018-0207-5
Wittenburg N, Baumeister R (1999) Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc Natl Acad Sci U S A 96:10477–10482
Wortley MA, Birrell MA, Belvisi MG (2017) Drugs affecting TRP channels. Handb Exp Pharmacol 237:213–241. https://doi.org/10.1007/164_2016_63
Martins D, Silva M, Tavares I (2017) TRPV1 in pain control from the brain. Oncotarget 8:16101–16102. https://doi.org/10.18632/oncotarget.13316
Caterina MJ, Schumacher MA, Tominaga M et al (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Yang BH, Piao ZG, Kim Y-B et al (2016) Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res 82:781–785. https://doi.org/10.1177/154405910308201004
Yang BH, Piao ZG, Kim Y-B et al (2003) Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res 82:781–785
Ohkubo T, Shibata M (1997) The selective capsaicin antagonist capsazepine abolishes the antinociceptive action of eugenol and guaiacol. J Dent Res 76:848–851. https://doi.org/10.1177/00220345970760040501
Jancsó G, Dux M, Oszlács O, Sántha P (2008) Activation of the transient receptor potential vanilloid-1 (TRPV1) channel opens the gate for pain relief. Brit J Pharmacol 155:1139–1141. https://doi.org/10.1038/bjp.2008.375
Fernández-Ballester G, Ferrer-Montiel A (2008) Molecular modeling of the full-length human TRPV1 channel in closed and desensitized states. J Membr Biol 223:161–172. https://doi.org/10.1007/s00232-008-9123-7
Elokely K, Velisetty P, Delemotte L et al (2016) Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin. Proc Natl Acad Sci 113:E137–E145. https://doi.org/10.1073/pnas.1517288113
Ikenaka K, Tsukada Y, Giles AC et al (2019) A behavior-based drug screening system using a Caenorhabditis elegans model of motor neuron disease. Sci Rep 9:10104. https://doi.org/10.1038/s41598-019-46642-6
Martinez BA, Caldwell KA, Caldwell GA (2017) C. elegans as a model system to accelerate discovery for Parkinson disease. Curr Opin Genet Dev 44:102–109. https://doi.org/10.1016/j.gde.2017.02.011
Markaki M, Tavernarakis N (2020) Caenorhabditis elegans as a model system for human diseases. Curr Opin Biotechnol 63:118–125. https://doi.org/10.1016/j.copbio.2019.12.011
Schmeisser K, Parker JA (2018) Worms on the spectrum - C. elegans models in autism research. Exp Neurol 299:199–206. https://doi.org/10.1016/j.expneurol.2017.04.007
Mohammadi A, Rodgers JB, Kotera I, Ryu WS (2013) Behavioral response of Caenorhabditis elegans to localized thermal stimuli. BMC Neurosci 14:66. https://doi.org/10.1186/1471-2202-14-66
Srinivasan J, Durak O, Sternberg PW (2008) Evolution of a polymodal sensory response network. BMC Biol 6:52. https://doi.org/10.1186/1741-7007-6-52
Lindy AS, Parekh PK, Zhu R et al (2014) TRPV channel-mediated calcium transients in nociceptor neurons are dispensable for avoidance behaviour. Nat Commun 5:4734. https://doi.org/10.1038/ncomms5734
Liedtke WB, Heller S, Sze JY (2007) The TRPV channel in C. elegans serotonergic neurons. CRC Press, Boca Raton
Kahn-Kirby AH, Bargmann CI (2006) TRP channels in C. elegans. Annu Rev Physiol 68:719–736. https://doi.org/10.1146/annurev.physiol.68.040204.100715
Montell C (2003) The venerable inveterate invertebrate TRP channels. Cell Calcium 33:409–417
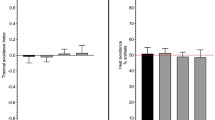
Nkambeu B, Salem JB, Beaudry F (2020) Capsaicin and its analogues impede nocifensive response of caenorhabditis elegans to noxious heat. Neurochem Res. https://doi.org/10.1007/s11064-020-03049-4
Nkambeu B, Salem JB, Beaudry F (2021) Eugenol and other vanilloids hamper caenorhabditis elegans response to noxious heat. Neurochem Res 46:252–264. https://doi.org/10.1007/s11064-020-03159-z
Perrin J, Werner T, Kurzawa N et al (2020) Identifying drug targets in tissues and whole blood with thermal-shift profiling. Nat Biotechnol 38:303–308. https://doi.org/10.1038/s41587-019-0388-4
Hong KT, Lee J-S (2020) Label-free proteome profiling as a quantitative target identification technique for bioactive small molecules. Biochemistry-us 59:213–215. https://doi.org/10.1021/acs.biochem.9b00975
Kaur U, Meng H, Lui F et al (2018) Proteome-wide structural biology: an emerging field for the structural analysis of proteins on the proteomic scale. J Proteome Res 17:3614–3627. https://doi.org/10.1021/acs.jproteome.8b00341
Mateus A, Määttä TA, Savitski MM (2017) Thermal proteome profiling: unbiased assessment of protein state through heat-induced stability changes. Proteome sci. https://doi.org/10.1186/s12953-017-0122-4
Margie O, Palmer C, Chin-Sang I (2013) Chemotaxis assay. J vis exp. https://doi.org/10.3791/50069
Brenner S (1974) The genetics of caenorhabditis elegans. Genetics 77:71–94. https://doi.org/10.1093/genetics/77.1.71
Ohnishi K, Saito S, Miura T et al (2020) OSM-9 and OCR-2 TRPV channels are accessorial warm receptors in Caenorhabditis elegans temperature acclimatisation. Sci Rep-uk 10:18566. https://doi.org/10.1038/s41598-020-75302-3
Zhou Y, Zhou B, Pache L et al (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10:1523. https://doi.org/10.1038/s41467-019-09234-6
Bindea G, Mlecnik B, Hackl H et al (2009) ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25:1091–1093. https://doi.org/10.1093/bioinformatics/btp101
Shannon P, Markiel A, Ozier O et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B Methodol 57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
Salem JB, Nkambeu B, Arvanitis DN, Beaudry F (2022) Resiniferatoxin hampers the nocifensive response of caenorhabditis elegans to noxious heat, and pathway analysis revealed that the Wnt signaling pathway is involved. Neurochem Res 47:622–633. https://doi.org/10.1007/s11064-021-03471-2
Gupta N, Pevzner PA (2009) False discovery rates of protein identifications: a strike against the two-peptide rule. J Proteome Res 8:4173–4181. https://doi.org/10.1021/pr9004794
Iftinca M, Defaye M, Altier C (2021) TRPV1-targeted drugs in development for human pain conditions. Drugs 81:7–27. https://doi.org/10.1007/s40265-020-01429-2
Sultana A, Singla RK, He X et al (2021) Topical capsaicin for the treatment of neuropathic pain. Curr Drug Metab 22:198–207. https://doi.org/10.2174/1389200221999201116143701
Leavell Y, Simpson DM (2022) The role of the capsaicin 8% patch in the treatment of painful diabetic peripheral neuropathy. Pain Manag 12:595–609. https://doi.org/10.2217/pmt-2021-0025
Giaccari LG, Aurilio C, Coppolino F et al (2021) Capsaicin 8% patch and chronic postsurgical neuropathic pain. J Pers Med 11:960. https://doi.org/10.3390/jpm11100960
Ausín-Crespo MD, Castro EM, Roldán-Cuartero J et al (2022) Capsaicin 8% dermal patch for neuropathic pain in a pain unit. Pain Manag Nurs. https://doi.org/10.1016/j.pmn.2021.12.005
Roeckel L-A, Coz G-ML, Gavériaux-Ruff C, Simonin F (2016) Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 338:160–182. https://doi.org/10.1016/j.neuroscience.2016.06.029
Guichard L, Hirve A, Demiri M, Martinez V (2021) Opioid-induced hyperalgesia in patients with chronic pain: a systematic review of published cases. Clin J Pain 38:49–57. https://doi.org/10.1097/ajp.0000000000000994
Svensson CK (2022) Opioid-induced hyperalgesia: is it a clinically relevant phenomenon? Int J Pharm Pract. https://doi.org/10.1093/ijpp/riac031
Zhang Y-K, Huang Z-J, Liu S et al (2013) WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest 123:2268–2286. https://doi.org/10.1172/jci65364
Zhou Y-Q, Tian X-B, Tian Y-K et al (2022) Wnt signaling: a prospective therapeutic target for chronic pain. Pharmacol Therapeut 231:107984. https://doi.org/10.1016/j.pharmthera.2021.107984
Itokazu T, Hayano Y, Takahashi R, Yamashita T (2014) Involvement of Wnt/β-catenin signaling in the development of neuropathic pain. Neurosci Res 79:34–40. https://doi.org/10.1016/j.neures.2013.12.002
Han B, Zhao J, Wang W et al (2017) Cdc42 promotes schwann cell proliferation and migration through Wnt/β-catenin and p38 MAPK signaling pathway after sciatic nerve injury. Neurochem Res 42:1317–1324. https://doi.org/10.1007/s11064-017-2175-2
Gernez Y, de Jesus AA, Alsaleem H et al (2019) Severe autoinflammation in 4 patients with C-terminal variants in cell division control protein 42 homolog (CDC42) successfully treated with IL-1β inhibition. J Allergy Clin Immun 144:1122-1125.e6. https://doi.org/10.1016/j.jaci.2019.06.017
Zhou WBS, Shi XQ, Liu Y et al (2022) Unbiased proteomic analysis detects painful systemic inflammatory profile in the serum of nerve injured mice. Pain. https://doi.org/10.1097/j.pain.0000000000002695
Mazumder B, Sampath P, Seshadri V et al (2003) Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell 115:187–198. https://doi.org/10.1016/s0092-8674(03)00773-6
Chédotal A (2019) Roles of axon guidance molecules in neuronal wiring in the develo** spinal cord. Nat Rev Neurosci 20:380–396. https://doi.org/10.1038/s41583-019-0168-7
Ke C, Gao F, Tian X et al (2017) Slit2/Robo1 mediation of synaptic plasticity contributes to bone cancer pain. Mol Neurobiol 54:295–307. https://doi.org/10.1007/s12035-015-9564-9
Llorián-Salvador M, González-Rodríguez S (2018) Painful Understanding of VEGF. Front Pharmacol 9:1267. https://doi.org/10.3389/fphar.2018.01267
den Eynde CV, Vriens J, Clercq KD (2021) Transient receptor potential channel regulation by growth factors. Biochimica Et Biophysica Acta Bba - Mol Cell Res 1868:118950. https://doi.org/10.1016/j.bbamcr.2021.118950
Acknowledgements
This project was funded by the National Sciences and Engineering Research Council of Canada (F. Beaudry discovery Grant Number RGPIN-2020-05228). Laboratory equipment was funded by the Canadian Foundation for Innovation (CFI) and the Fonds de Recherche du Québec (FRQ), the Government of Quebec (F. Beaudry CFI John R. Evans Leaders Grant Number 36706). F. Beaudry is the holder of the Canada Research Chair in metrology of bioactive molecule and target discovery (Grant Number CRC-2021-00160). This research was undertaken partly thanks to funding from the Canada Research Chairs Program. A Ph.D. scholarship was awarded to J. Ben Salem from the Fonds de recherche du Québec—Santé (FRQS).
Funding
This study was supported by Natural Sciences and Engineering Research Council of Canada, RGPIN-2020-05228, Canadian Foundation for Innovation, 36706, Canada Research Chairs, CRC-2021-00160.
Author information
Authors and Affiliations
Contributions
BN: Planning and Execution of experiments, Writing - original draf. JBS: Execution of experiments, Writing - review & editing. FB: Conceptualization, Funding, Organisation, Writing - review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nkambeu, B., Salem, J.B. & Beaudry, F. Antinociceptive Activity of Vanilloids in Caenorhabditis elegans is Mediated by the Desensitization of the TRPV Channel OCR-2 and Specific Signal Transduction Pathways. Neurochem Res 48, 1900–1911 (2023). https://doi.org/10.1007/s11064-023-03876-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03876-1