Abstract
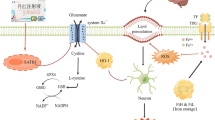
Cerebral ischemia reperfusion injury (CIRI) is the commonest cause of brain dysfunction. Up-regulation of POU domain class 2 transcription factor 2 (POU2F2) has been reported in patients with cerebral ischemia, while the role of POU2F2 in CIRI remains elusive. Middle cerebral artery occlusion/reperfusion (MCAO/R) in mice and oxygen and glucose deprivation/reperfusion (OGD/R) in mouse primary cortical neurons were used as models of CIRI injury in vivo and in vitro. Lentivirus-mediated POU2F2 knockdown further impaired CIRI induced by MCAO/R in mice, which was accompanied by increased-neurological deficits, cerebral infarct volume and neuronal loss. Our evidence suggested that POU2F2 deficiency deteriorated oxidative stress and ferroptosis according to the phenomenon such as the abatement of SOD, GSH, glutathione peroxidase 4 (GPX4) activity and accumulation of ROS, lipid ROS, 4-hydroxynonenal (4-HNE) and MDA. In vivo, primary cortical neurons with POU2F2 knockdown also showed worse neuronal damage, oxidative stress and ferroptosis. Sestrin2 (Sesn2) was reported as a neuroprotection gene and involved in ferroptosis mechanism. Up-regulation of Sesn2 was observed in the ischemic penumbra and OGD/R-induced neuronal cells. Further, we proved that POU2F2, as a transcription factor, could bind to Sesn2 promoter and positively regulate its expression. Sesn2 overexpression relieved oxidative stress and ferroptosis induced by POU2F2 knockdown in OGD/R-treated neurons. This research demonstrated that CIRI induced a compensatory increase of POU2F2 and Sesn2. Down-regulated POU2F2 exacerbated CIRI through the acceleration of oxidative stress and ferroptosis possibly by decreasing Sesn2 expression, which offers new sights into therapeutic mechanisms for CIRI.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sacco RL, Rundek T (2012) Cerebrovascular disease. Curr Opin Neurol 25(1):1–4. https://doi.org/10.1097/WCO.0b013e32834f89b1
Liu W, Wong A, Law AC et al (2015) Cerebrovascular disease, amyloid plaques, and dementia. Stroke 46(5):1402–1407. https://doi.org/10.1161/strokeaha.114.006571
Catanese L, Tarsia J, Fisher M (2017) Acute ischemic stroke therapy overview. Circ Res 120(3):541–558. https://doi.org/10.1161/circresaha.116.309278
Frizzell JP (2005) Acute stroke: pathophysiology, diagnosis, and treatment. AACN Clin Issues 16(4):421–440 quiz 597-8. https://doi.org/10.1097/00044067-200510000-00002
Sanderson TH, Reynolds CA, Kumar R et al (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47(1):9–23. https://doi.org/10.1007/s12035-012-8344-z
Nagy Z, Nardai S (2017) Cerebral ischemia/repefusion injury: from bench space to bedside. Brain Res Bull. https://doi.org/10.1016/j.brainresbull.2017.06.011
Patel RAG, McMullen PW (2017) Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis 59(6):542–548. https://doi.org/10.1016/j.pcad.2017.04.005
Meng X, **e W (2018) Neuroprotective effects of radix scrophulariae on cerebral ischemia and reperfusion injury via MAPK pathways. Molecules. https://doi.org/10.3390/molecules23092401
Zhang Y, Zhang Y, ** XF et al (2019) The role of astragaloside IV against cerebral ischemia/reperfusion injury: suppression of apoptosis via promotion of P62-LC3-autophagy. Molecules. https://doi.org/10.3390/molecules24091838
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Hassannia B, Vandenabeele P, Vanden Berghe T (2019) Targeting ferroptosis to iron out cancer. Cancer Cell 35(6):830–849. https://doi.org/10.1016/j.ccell.2019.04.002
**e Y, Hou W, Song X et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379. https://doi.org/10.1038/cdd.2015.158
Stockwell BR, Friedmann Angeli JP, Bayir H et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285. https://doi.org/10.1016/j.cell.2017.09.021
Skouta R, Dixon SJ, Wang J et al (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136(12):4551–4556. https://doi.org/10.1021/ja411006a
Alim I, Caulfield JT, Chen Y et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262–1279
She X, Lan B, Tian H et al (2020) Cross talk between ferroptosis and cerebral ischemia. Front Neurosci 14:776. https://doi.org/10.3389/fnins.2020.00776
Budanov AV, Lee JH, Karin M (2010) Stressin’ Sestrins take an aging fight. EMBO Mol Med 2(10):388–400. https://doi.org/10.1002/emmm.201000097
Pasha M, Eid AH (2017) Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid Med Cell Longev. https://doi.org/10.1155/2017/3296294
Wang P, Zhao Y, Li Y et al (2019) Sestrin2 overexpression attenuates focal cerebral ischemic injury in rat by increasing Nrf2/HO-1 pathway-mediated angiogenesis. Neuroscience 410:140–149. https://doi.org/10.1016/j.neuroscience.2019.05.005
Li L, **ao L, Hou Y et al (2016) Sestrin2 silencing exacerbates cerebral ischemia/reperfusion injury by decreasing mitochondrial biogenesis through the AMPK/PGC-1α pathway in rats. Sci Rep 6:30272. https://doi.org/10.1038/srep30272
Li JY, Ren C, Wang LX et al (2021) Sestrin2 protects dendrite cells against ferroptosis induced by sepsis. Cell Death Dis 12:834. https://doi.org/10.1038/s41419-021-04122-8
Staudt LM, Clerc RG, Singh H et al (1988) Cloning of a lymphoid-specific cDNA encoding a protein binding the regulatory octamer DNA motif. Science 241(4865):577–580. https://doi.org/10.1126/science.3399892
Hodson DJ, Shaffer AL, **ao W et al (2016) Regulation of normal B-cell differentiation and malignant B-cell survival by OCT2. Proc Natl Acad Sci USA 113(14):E2039–E2046. https://doi.org/10.1073/pnas.1600557113
Latchman DS (1996) The Oct-2 transcription factor. Int J Biochem Cell Biol 28(10):1081–1083. https://doi.org/10.1016/1357-2725(96)00050-7
Latchman DS (1996) Activation and repression of gene expression by POU family transcription factors. Philos Trans R Soc Lond B 351(1339):511–515. https://doi.org/10.1098/rstb.1996.0049
Camós S, Gubern C, Sobrado M et al (2014) Oct-2 transcription factor binding activity and expression up-regulation in rat cerebral ischaemia is associated with a diminution of neuronal damage in vitro. Neuromolecular Med 16(2):332–349. https://doi.org/10.1007/s12017-013-8279-1
Lelièvre E, Lionneton F, Soncin F et al (2001) The Ets family contains transcriptional activators and repressors involved in angiogenesis. Int J Biochem Cell Biol 33(4):391–407. https://doi.org/10.1016/s1357-2725(01)00025-5
Ikeshima H, Imai S, Shimoda K et al (1995) Expression of a MADS box gene, MEF2D, in neurons of the mouse central nervous system: implication of its binary function in myogenic and neurogenic cell lineages. Neurosci Lett 200(2):117–120. https://doi.org/10.1016/0304-3940(95)12092-i
Crack PJ, Taylor JM (2005) Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 38(11):1433–1444. https://doi.org/10.1016/j.freeradbiomed.2005.01.019
Li M, Ma Y, Zhong Y et al (2020) KALRN mutations promote antitumor immunity and immunotherapy response in cancer. J ImmunoTher Cancer. https://doi.org/10.1136/jitc-2019-000293
McCullough LD, Blizzard K, Simpson ER et al (2003) Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci 23(25):8701–8705. https://doi.org/10.1523/jneurosci.23-25-08701.2003
Chen F, Zhang L, Wang E et al (2018) LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochem Biophys Res Commun 496(1):184–190. https://doi.org/10.1016/j.bbrc.2018.01.022
Li X, **a Q (2021) Annexin-A1 SUMOylation regulates microglial polarization after cerebral ischemia by modulating IKKα stability via selective autophagy. Sci Adv. https://doi.org/10.1126/sciadv.abc5539
Liu H, Li Y, Sun S et al (2021) Catalytically potent and selective clusterzymes for modulation of neuroinflammation through single-atom substitutions. Nat Commun 12:114. https://doi.org/10.1038/s41467-020-20275-0
Conrad M, Angeli JP, Vandenabeele P et al (2016) Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 15(5):348–366. https://doi.org/10.1038/nrd.2015.6
Longa EZ, Weinstein PR, Carlson S et al (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91. https://doi.org/10.1161/01.str.20.1.84
Guo P, ** Z, Wu H et al (2019) Effects of irisin on the dysfunction of blood-brain barrier in rats after focal cerebral ischemia/reperfusion. Brain Behav 9(10):e01425. https://doi.org/10.1002/brb3.1425
Bakheet SA, Basha MR, Cai H et al (2007) Lead exposure: expression and activity levels of Oct-2 in the develo** rat brain. Toxicol Sci 95(2):436–442. https://doi.org/10.1093/toxsci/kfl163
Gottesman RF, Hillis AE (2010) Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol 9(9):895–905. https://doi.org/10.1016/s1474-4422(10)70164-2
Sun MS, ** H, Sun X et al (2018) Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. https://doi.org/10.1155/2018/3804979
Pan J, Konstas AA, Bateman B et al (2007) Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 49(2):93–102. https://doi.org/10.1007/s00234-006-0183-z
Wang P, Cui Y, Ren Q et al (2021) Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis 12:447. https://doi.org/10.1038/s41419-021-03725-5
Li X, Ma N, Xu J et al (2021) Targeting ferroptosis: pathological mechanism and treatment of ischemia-reperfusion injury. Oxid Med Cell Longiv. https://doi.org/10.1155/2021/1587922
Li N, Wang W, Zhou H et al (2020) Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. https://doi.org/10.1016/j.freeradbiomed.2020.08.009
Yamada N, Karasawa T, Kimura H (2020) Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Discov 11:144. https://doi.org/10.1038/s41419-020-2334-2
Galaris D, Barbouti A, Pantopoulos K (2019) Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys Acta Mol Cell Res 1866(12):118535. https://doi.org/10.1016/j.bbamcr.2019.118535
Cao JY, Dixon SJ (2016) Mechanisms of ferroptosis. Cell Mol Life Sci 73(11–12):2195–2209. https://doi.org/10.1007/s00018-016-2194-1
Fionda C, Di Bona D, Kosta A et al (2019) The POU-domain transcription factor Oct-6/POU3F1 as a regulator of cellular response to genotoxic stress. Cancers (Basel). https://doi.org/10.3390/cancers11060810
Budanov AV, Sablina AA, Feinstein E et al (2004) Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304(5670):596–600. https://doi.org/10.1126/science.1095569
Park SJ, Cho SS, Kim KM et al (2019) Protective effect of sestrin2 against iron overload and ferroptosis-induced liver injury. Toxicol Appl Pharmacol. https://doi.org/10.1016/j.taap.2019.114665
Funding
This research was supported by grants from the Youth Fund Project of National Natural Science Foundation of China (Grant Number 82001226) and the Natural Science Foundation of Jilin Province (Grant Number 20210101357JC).
Author information
Authors and Affiliations
Contributions
Conceptualization: JY, SY; methodology: JY, QG, LW, SY; writing—original draft preparation: JY; writing—review and editing: SY; funding acquisition: SY.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
The animal experiment in this study was approved by the ethical guideline of the Animal Welfare and Research Ethics Committee of Jilin University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Guo, Q., Wang, L. et al. POU Domain Class 2 Transcription Factor 2 Inhibits Ferroptosis in Cerebral Ischemia Reperfusion Injury by Activating Sestrin2. Neurochem Res 48, 658–670 (2023). https://doi.org/10.1007/s11064-022-03791-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03791-x