Abstract
Focal ischemic stroke (FIS) is a leading cause of human debilitation and death. Following the onset of a FIS, the brain experiences a series of spatiotemporal changes which are exemplified in different pathological processes. One prominent feature of FIS is the development of reactive astrogliosis and glial scar formation in the peri-infarct region (PIR). During the subacute phase, astrocytes in PIR are activated, referred to as reactive astrocytes (RAs), exhibit changes in morphology (hypotrophy), show an increased proliferation capacity, and altered gene expression profile, a phenomenon known as reactive astrogliosis. Subsequently, the morphology of RAs remains stable, and proliferation starts to decline together with the formation of glial scars. Reactive astrogliosis and glial scar formation eventually cause substantial tissue remodeling and changes in permanent structure around the PIR. Glial cell line-derived neurotrophic factor (GDNF) was originally isolated from a rat glioma cell-line and regarded as a potent survival neurotrophic factor. Under normal conditions, GDNF is expressed in neurons but is upregulated in RAs after FIS. This review briefly describes properties of GDNF, its receptor-mediated signaling pathways, as well as recent studies regarding the role of RAs-derived GDNF in neuronal protection and brain recovery. These results provide evidence suggesting an important role of RA-derived GDNF in intrinsic brain repair and recovery after FIS, and thus targeting GDNF in RAs may be effective for stroke therapy.

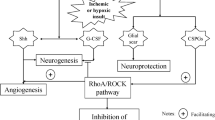
Adapted from reference Li et al.

Adapted from Kramer and Liss

Similar content being viewed by others
References
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–397
Dirnagl U (2012) Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci 1268:21–25
Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26:248–254
Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA (2019) Ischaemic stroke. Nat Rev Dis Primers 5:70
Verkhratsky A, Nedergaard M (2017) Physiology of astroglia. Physiol Rev 98:239–389
Halassa MM, Fellin T, Haydon PG (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13:54–63
Haydon PG (2001) GLIA: listening and talking to the synapse. [Review] [90 refs]. Nat Rev Neurosci 2:185–193
Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86:1009–1031
Kimelberg H, Nedergaard M (2010) Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7:338–353
Zuchero JB, Barres BA (2015) Glia in mammalian development and disease. Development 142:3805–3809
Pekny M, Pekna M, Messing A, Steinhñuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345
Verkhratsky A, Zorec R, Parpura V (2017) Stratification of astrocytes in healthy and diseased brain. Brain Pathol 27:629–644
Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH (2009) In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci 29:1719–1734
Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S (2013) Multimodal examination of structural and functional remap** in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab 33:716–723
Murphy TH, Corbett D (2009) Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10:861–872
Mostany R, Chowdhury TG, Johnston DG, Portonovo SA, Carmichael ST, Portera-Cailliau C (2010) Local hemodynamics dictate long-term dendritic plasticity in peri-infarct cortex. J Neurosci 30:14116–14126
Winship IR, Murphy TH (2008) In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. J Neurosci 28:6592–6606
Barreto GE, Sun X, Xu L, Giffard RG (2011) Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS ONE 6:e27881
Li H et al (2014) Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neurosci 15:58
Choudhury GR, Ding S (2016) Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis 85:234–244
Ding S (2014) Dynamic reactive astrocytes after focal ischemia. Neural Regenerative Res 9:2048–2052
Burda J, Sofroniew M (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522
Linnerbauer M, Rothhammer V (2020) Protective functions of reactive astrocytes following central nervous system insult. Front Immunol 11:2571
Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV (2006) Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129:2761–2772
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155
Anderson MA, Burda JE, Ren Y, Ao Y, Ö’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410
Zhao Y, Rempe D (2010) Targeting astrocytes for stroke therapy. Neurotherapeutics 7:439–451
Gleichman AJ, Carmichael ST (2014) Astrocytic therapies for neuronal repair in stroke. Neurosci Lett 565:47–52
Chouchane M, Costa MR (2012) Cell therapy for stroke: use of local astrocytes. Front Cell Neurosci 6:49
Escartin C, Bonvento G (2008) Targeted activation of astrocytes: a potential neuroprotective strategy. Mol Neurobiol 38:231–241
Lobsiger CS, Cleveland DW (2007) Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 10:1355–1360
Jolly S, Journiac N, Naudet FDR, Gautheron V, Mariani J, Vernet-der Garabedian B (2011) Cell-autonomous and non-cell-autonomous neuroprotective functions of RORα in neurons and astrocytes during hypoxia. J Neurosci 31:14314–14323
Li H, **e Y, Zhang N, Yu Y, Zhang Q, Ding S (2015) Disruption of IP3R2-mediated Ca2+ signaling pathway in astrocytes ameliorates neuronal death and brain damage while reducing behavioral deficits after focal ischemic stroke. Cell Calcium 58:565–576
Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neuro 2:679–689
Pecho-Vrieseling E, Rieker C, Fuchs S, Bleckmann D, Esposito MS, Botta P, Goldstein C, Bernhard M, Galimberti I, Muller M, Luthi A, Arber S, Bouwmeester T, van der Putten H, Di Giorgio FP (2014) Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat Neurosci 17:1064–1072
Barreto GE, Gonzalez J, Torres Y, Morales L (2011) Astrocytic-neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci Res 71:107–113
Becerra-Calixto A, Cardona-Gomez GP (2017) The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci 10:88
Poyhonen S, Er S, Domanskyi A, Airavaara M (2019) Effects of neurotrophic factors in glial cells in the central nervous system: expression and properties in neurodegeneration and injury. Front Physiol 10:486
Duarte ELP, Curcio M, Canzoniero LM, Duarte CB (2012) Neuroprotection by GDNF in the ischemic brain. Growth Factors 30:242–257
Harvey BK, Hoffer BJ, Wang Y (2005) Stroke and TGF-α proteins: glial cell line-derived neurotrophic factor and bone morphogenetic protein. Pharmacol Ther 105:113–125
Nakajima K, Hida H, Shimano Y, Fujimoto I, Hashitani T, Kumazaki M, Sakurai T, Nishino H (2001) GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Res 916:76–84
Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF (2007) GDNF and GFRα1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci 10:293–300
Chen Y, Ai Y, Slevin JR, Maley BE, Gash DM (2005) Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Exp Neurol 196:87–95
Airaksinen MS, Saarma M (2002) The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394
Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J (2008) Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 11:755–761
Uesaka T, Nagashimada M, Enomoto H (2013) GDNF signaling levels control migration and neuronal differentiation of enteric ganglion precursors. J Neurosci 33:16372–16382
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A (1996) Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev 54:95–105
Cortes D, Carballo-Molina OA, Castellanos-Montiel MAJ, Velasco I (2017) The non-survival effects of glial cell line-derived neurotrophic factor on neural cells. Front Mol Neurosci 10:258
Cortes D, Robledo-Arratia Y, Hernandez-Martinez R, Escobedo-Avila I, Bargas J, Velasco I (2016) Transgenic GDNF positively influences proliferation, differentiation, maturation and survival of motor neurons produced from mouse embryonic stem cells. Front Cell Neurosci 10:217
Bespalov MM, Sidorova YA, Tumova S, Ahonen-Bishopp A, Magalhaes AC, Kulesskiy E, Paveliev M, Rivera C, Rauvala H, Saarma M (2011) Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol 192:153–169
Saavedra A, Baltazar G, Duarte ELP (2008) Driving GDNF expression: the green and the red traffic lights. Progr Neurobiol 86:186–215
Hisaoka K, Nishida A, Koda T, Miyata M, Zensho H, Morinobu S, Ohta M, Yamawaki S (2001) Antidepressant drug treatments induce glial cell line-derived neurotrophic factor (GDNF) synthesis and release in rat C6 glioblastoma cells. J Neurochem 79:25–34
Tanabe K, Matsushima-Nishiwaki R, Iida M, Kozawa O, Iida H (2012) Involvement of phosphatidylinositol 3-kinase/Akt on basic fibroblast growth factor-induced glial cell line-derived neurotrophic factor release from rat glioma cells. Brain Res 1463:21–29
Verity AN, Wyatt TL, Hajos B, Eglen RM, Baecker PA, Johnson RM (1998) Regulation of glial cell line-derived neurotrophic factor release from rat C6 glioblastoma cells. J Neurochem 70:531–539
Tanabe K, Nishimura K, Dohi S, Kozawa O (2009) Mechanisms of interleukin-1β-induced GDNF release from rat glioma cells. Brain Res 1274:11–20
Kuno R, Yoshida Y, Nitta A, Nabeshima T, Wang J, Sonobe Y, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A (2006) The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res 1116:12–18
Lonka-Nevalaita L, Lume M, Leppanen S, Jokitalo E, Peranen J, Saarma M (2010) Characterization of the intracellular localization, processing, and secretion of two glial cell line-derived neurotrophic factor splice isoforms. J Neurosci 30:11403
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155–175
Wang X (2013) Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochimica et Biophysica Acta (BBA) 1834:2205–2212
Sariola H, Saarma M (2003) Novel functions and signalling pathways for GDNF. J Cell Sci 116:3855
Ibanez CF, Andressoo JO (2017) Biology of GDNF and its receptors-relevance for disorders of the central nervous system. Neurobiol Dis 97:80–89
Barnett MW, Fisher CE, Perona-Wright G, Davies JA (2002) Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J Cell Sci 115:4495
Eketjall S, Fainzilber M, Murray-Rust J, Ibanez CF (1999) Distinct structural elements in GDNF mediate binding to GFRalpha1 and activation of the GFR-c-Ret receptor complex. EMBO J 18:5901–5910
Trupp M, Belluardo N, Funakoshi H, Ibanez CF (1997) Complementary and overlap** expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-α indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci 17:3554
Paratcha G, Ledda F, Ibanez CF (2003) The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113:867–879
Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39
Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM (2002) Lipid rafts in neuronal signaling and function. Trends Neurosci 25:412–417
Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, Arumñe U, Saarma M (1999) GDNF triggers a novel Ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor α1. FEBS Lett 463:63–66
Baloh RH, Enomoto H, Johnson EM, Milbrandt J (2000) The GDNF family ligands and receptors-implications for neural development. Curr Opin Neurobiol 10:103–110
Takahashi M (2001) The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 12:361–373
Coulpier M, Anders J, Ibanez CF (2002) Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem 277:1991–1999
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134
Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF (2001) Released GFRα1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron 29:171–184
Ibanez CF (2013) Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harbor Perspect Biol 5:a009134
Chen ZY, Chai YF, Cao L, Huang AJ, Cui RY, Lu CL, He C (2001) Glial cell line-derived neurotrophic factor promotes survival and induces differentiation through the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathway respectively in PC12 cells. Neuroscience 104:593–598
Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM (2001) c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci 21:1464
Yang F, He XP, Feng L, Mizuno K, Liu XW, Russell J, **ong WC, Lu B (2001) PI-3 kinase and IP3 are both necessary and sufficient to mediate NT3-induced synaptic potentiation. Nat Neurosci 4:19–28
Fukuda T, Kiuchi K, Takahashi M (2002) Novel mechanism of regulation of RAC activity and lamellipodia formation by RET tyrosine kinase. J Biol Chem 277:19114–19121
Richardson DS, Rodrigues DM, Hyndman BD, Crupi MJF, Nicolescu AC, Mulligan LM (2012) Alternative splicing results in RET isoforms with distinct trafficking properties. MBoC 23:3838–3850
de Graaff E, Srinivas S, Kilkenny C, D’Agati V, Mankoo BS, Costantini F, Pachnis V (2001) Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev 15:2433–2444
Trupp M, Scott R, Whittemore SR, Ibanez CF (1999) Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem 274:20885–20894
Luz M, Mohr E, Fibiger HC (2016) GDNF-induced cerebellar toxicity: a brief review. NeuroToxicology 52:46–56
Kirik D, Georgievska B, Bjorklund A (2004) Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci 7:105–110
Yamagata K, Hakata K, Maeda A, Mochizuki C, Matsufuji H, Chino M, Yamori Y (2007) Adenosine induces expression of glial cell line-derived neurotrophic factor (GDNF) in primary rat astrocytes. Neurosci Res 59:467–474
Rocha SM, Cristovao AC, Campos FL, Fonseca CP, Baltazar G (2012) Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol Dis 47:407–415
Yan M, Dai H, Ding T, Dai A, Zhang F, Yu L, Chen G, Chen Z (2011) Effects of dexmedetomidine on the release of glial cell line-derived neurotrophic factor from rat astrocyte cells. Neurochem Int 58:549–557
Chen SH, Oyarzabal EA, Sung YF, Chu CH, Wang Q, Chen SL, Lu RB, Hong JS (2015) Microglial regulation of immunological and neuroprotective functions of astroglia. GLIA 63:118–131
Foo L, Allen N, Bushong E, Ventura P, Chung WS, Zhou L, Cahoy J, Daneman R, Zong H, Ellisman M, Barres B (2011) Development of a method for the purification and culture of rodent astrocytes. Neuron 71:799–811
Miyazaki H, Nagashima K, Okuma Y, Nomura Y (2001) Expression of glial cell line-derived neurotrophic factor induced by transient forebrain ischemia in rats. Brain Res 922:165–172
Hidalgo-Figueroa MA, Bonilla S, Gutierrez F, Pascual A, Lopez-Barneo J (2012) GDNF is predominantly expressed in the PV+ neostriatal interneuronal ensemble in normal mouse and after injury of the nigrostriatal pathway. J Neurosci 32:864–872
Kuric E, Wieloch T, Ruscher K (2013) Dopamine receptor activation increases glial cell line-derived neurotrophic factor in experimental stroke. Exp Neurol 247:202–208
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947
Abe K, Hayashi T (1997) Expression of the glial cell line-derived neurotrophic factor gene in rat brain after transient MCA occlusion. Brain Res 776:230–234
Kitagawa H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Warita H, Mitsumoto Y, Mori T, Abe K (1999) Induction of glial cell line-derived neurotrophic factor receptor proteins in cerebral cortex and striatum after permanent middle cerebral artery occlusion in rats. Brain Res 834:190–195
Wei GW, Wu GC, Cao XD (2000) Dynamic expression of glial cell line GÇÉ derived neurotrophic factor after cerebral ischemia. NeuroReport 11:1177–1183
Arvidsson A, Kokaia Z, Airaksinen MS, Saarma M, Lindvall O (2001) Stroke induces widespread changes of gene expression for glial cell line-derived neurotrophic factor family receptors in the adult rat brain. Neuroscience 106:27–41
Bresjanac M, Antauer G (2000) Reactive astrocytes of the quinolinic acid-lesioned rat striatum express GFRα1 as well as GDNF in vivo. Exp Neurol 164:53–59
Zhang N, Zhang Z, He R, Li H, Ding S (2020) GLAST-CreERT2 mediated deletion of GDNF increases brain damage and exacerbates long-term stroke outcomes after focal ischemic stroke in mouse model. GLIA 68:2395–2414
Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie ET, Vivien D, Buisson A (2001) Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of nmda-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci 21:3024
Marco S, Canudas AM, Canals JM, Gavaldá N, Perez-Navarro E, Alberch J (2002) Excitatory amino acids differentially regulate the expression of GDNF, neurturin, and their receptors in the adult rat striatum. Exp Neurol 174:243–252
Widenfalk J, Lundstrmer K, Jubran M, Brene S, Olson L (2001) Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci 21:3457
Wang Y, Lin SZ, Chiou AL, Williams LR, Hoffer BJ (1997) Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci 17:4341–4348
Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O (2006) Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke 37:2361–2367
Kitagawa H, Hayashi T, Mitsumoto Y, Koga N, Itoyama Y, Abe K (1998) Reduction of ischemic brain injury by topical application of glial cell line-derived neurotrophic factor after permanent middle cerebral artery occlusion in rats. Stroke 29:1417–1422
** G, Omori N, Li F, Nagano I, Manabe Y, Shoji M, Abe K (2003) Protection against ischemic brain damage by GDNF affecting cell survival and death signals. Int Congr Ser 1252:221–231
Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2006) Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res 84:1495–1504
Shang J, Deguchi K, Yamashita T, Ohta Y, Zhang H, Morimoto N, Liu N, Zhang X, Tian F, Matsuura T, Funakoshi H, Nakamura T, Abe K (2010) Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J Neurosci Res 88:2197–2206
Kilic U, Kilic E, Dietz Gunnar PH, Bahr M (2003) Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke 34:1304–1310
Sawada H, Ibi M, Kihara T, Urushitani M, Nakanishi M, Akaike A, Shimohama S (2000) Neuroprotective mechanism of glial cell line-derived neurotrophic factor in mesencephalic neurons. J Neurochem 74:1175–1184
Wong LF, Scott Ralph G, Walmsley LE, Bienemann AS, Parham S, Kingsman SM, Uney JB, Mazarakis ND (2005) Lentiviral-mediated delivery of Bcl-2 or GDNF protects against excitotoxicity in the rat hippocampus. Mol Ther 11:89–95
Ho A, Gore AC, Weickert CS, Blum M (1995) Glutamate regulation of GDNF gene expression in the striatum and primary striatal astrocytes. NeuroReport 6:1454–1458
Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N (1999) Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem 72:2334–2344
Endres M, Laufs U, Liao JK, Moskowitz MA (2004) Targeting eNOS for stroke protection. Trends Neurosci 27:283–289
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4:399–415
Ikeda T, **a XY, **a YX, Ikenoue T, Han B, Choi BH (2000) Glial cell line-derived neurotrophic factor protects against ischemia/hypoxia-induced brain injury in neonatal rat. Acta Neuropathol 100:161–167
Wang Y, Chang CF, Morales M, Chiang YH, Hoffer J (2002) Protective effects of glial cell line-derived neurotrophic factor in ischemic brain injury. Ann N Y Acad Sci 962:423–437
Hwang S, Mruk K, Rahighi S, Raub AG, Chen CH, Dorn LE, Horikoshi N, Wakatsuki S, Chen JK, Mochly-Rosen D (2018) Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat Commun 9:4045
Mejias R, Villadiego J, Pintado CO, Vime PJ, Gao L, Toledo-Aral JJ, Echevarrea M, Lopez-Barneo J (2006) Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J Neurosci 26:4500
Kuehne A, Emmert H, Soehle J, Winnefeld M, Fischer F, Wenck H, Gallinat S, Terstegen L, Lucius R, Hildebrand J, Zamboni N (2015) Acute activation of oxidative pentose phosphate pathway as first-line response to oxidative stress in human skin cells. Mol Cell 59:359–371
Jeng W, Loniewska MM, Wells PG (2013) Brain glucose-6-phosphate dehydrogenase protects against endogenous oxidative DNA damage and neurodegeneration in aged mice. ACS Chem Neurosci 4:1123–1132
Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M (2009) Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol Dis 33:405–414
Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH (2008) Non-cell-autonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3:649–657
Yagi T, Jikihara I, Fukumura M, Watabe K, Ohashi T, Eto Y, Hara M, Maeda M (2000) Rescue of ischemic brain injury by adenoviral gene transfer of glial cell line-derived neurotrophic factor after transient global ischemia in gerbils. Brain Res 885:273–282
Harvey BK, Chang CF, Chiang YH, Bowers WJ, Morales M, Hoffer BJ, Wang Y, Federoff HJ (2003) HSV amplicon delivery of glial cell line-derived neurotrophic factor is neuroprotective against ischemic injury. Exp Neurol 183:47–55
Tsai TH, Chen SL, Chiang YH, Lin SZ, Ma HI, Kuo SW, Tsao YP (2000) Recombinant adeno-associated virus vector expressing glial cell line-derived neurotrophic factor reduces ischemia-induced damage. Exp Neurol 166:266–275
Arvidsson A, Kirik D, Lundberg C, Mandel RJ, Andsberg G, Kokaia Z, Lindvall O (2003) Elevated GDNF levels following viral vector-mediated gene transfer can increase neuronal death after stroke in rats. Neurobiol Dis 14:542–556
Hermann DM, Kilic E, Kugler S, Isenmann S, Bahr M (2001) Adenovirus-mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice. Neurobiol Dis 8:655–666
**e Y, Wang T, Sun GY, Ding S (2010) Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience 170:992–1003
Jahn HM, Scheller A, Kirchhoff F (2015) Genetic control of astrocyte function in neural circuits. Front Cell Neurosci 9:310
Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M (2013) Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16:580
Paukert M, Agarwal A, Cha J, Doze V, Kang J, Bergles D (2014) Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82:1263–1270
DeCarolis NA, Mechanic M, Petrik D, Carlton A, Ables JL, Malhotra S, Bachoo R, Gotz M, Lagace DC, Eisch AJ (2013) In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus 23:708–719
Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M (2008) Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. PNAS 105:3581–3586
Sirko S, Behrendt G, Johansson P, Tripathi P, Costa MR, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer I, Plesnila N, Staufenbiel M, Haass C, Snapyan M, Saghatelyan A, Tsai LH, Fischer A, Grobe K, Dimou L, Gotz M (2013) Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 12:426–439
Robel S, Berninger B, Gotz M (2011) The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12:88–104
Dimou LGT (2014) Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain. Physiol Rev 94:709–737
Shimada IS, LeComte MD, Granger JC, Quinlan NJ, Spees JL (2012) Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J Neurosci 32:7926–7940
LeComte MD, Shimada IS, Sherwin C, Spees JL (2015) Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. PNAS 112:8726–8731
Shimada IS, Borders A, Aronshtam A, Spees JL (2011) Proliferating reactive astrocytes are regulated by notch-1 in the peri-infarct area after stroke. Stroke 42:3231–3237
Gotz M, Sirko S, Beckers J, Irmler M (2015) Reactive astrocytes as neural stem or progenitor cells: in vivo lineage, in vitro potential, and genome-wide expression analysis. GLIA 63:1452–1468
Ohab JJ, Fleming S, Blesch A, Carmichael ST (2006) A neurovascular niche for neurogenesis after stroke. J Neurosci 26:13007–13016
Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EMK, Lindvall O, Kokaia Z, Frisen J (2014) A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 346:237–241
Lois C, Alvarez-Buylla A (1993) Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. PNAS 90:2074
Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J (1998) Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol 36:249–266
Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O (2014) Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab 34:1573–1584
Young CC, van der Harg JM, Lewis NJ, Brooks KJ, Buchan AM, Szele FG (2013) Ependymal ciliary dysfunction and reactive astrocytosis in a reorganized subventricular zone after stroke. Cercor 23:647–659
Lagace DC (2012) Does the endogenous neurogenic response alter behavioral recovery following stroke? Behav Brain Res 227:426–432
Smith MP, Cass WA (2007) GDNF reduces oxidative stress in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci Lett 412:259–263
Ronning OM, Guldvog B (1998) Outcome of subacute stroke rehabilitation: a randomized controlled trial. Stroke 29:779–784
Poulin VR, Korner-Bitensky N, Bherer L, Lussier M, Dawson DR (2016) Comparison of two cognitive interventions for adults experiencing executive dysfunction post-stroke: a pilot study. Disabil Rehabil 39:1–13
Kramer ER, Liss B (2015) GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett 589:3760–3772
Acknowledgements
This work was supported by the National Institute of Health [National Institute of Neurological Disorders and Stroke (NINDS) Grants R01NS069726 and R01NS094539 to SD] and the America Heart Association [Midwest Affiliate Grant-in-Aid (Grant No. 16GRNT31280014), and NCRG-IRG 16IRG27780023 to SD].
Author information
Authors and Affiliations
Contributions
ZZ-original draft preparation; GS-review and editing; SD-review, editing and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honor of Prof. Vladimir Parpura.
Rights and permissions
About this article
Cite this article
Zhang, Z., Sun, G.Y. & Ding, S. Glial Cell Line-Derived Neurotrophic Factor and Focal Ischemic Stroke. Neurochem Res 46, 2638–2650 (2021). https://doi.org/10.1007/s11064-021-03266-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03266-5