Abstract
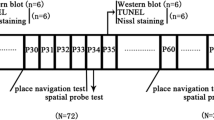
We aimed to observe the therapeutic effects of lithium on inhalational anesthetic sevoflurane-induced apoptosis in immature brain hippocampus. From postnatal day 5 (P5) to P28, male Sprague–Dawley pups were intraperitoneally injected with lithium chloride or 0.9 % sodium chloride. On P7 after the injection, pups were exposed to 2.3 % sevoflurane or air for 6 h. Brain tissues were harvested 12 h and 3 weeks after exposure. Cleaved caspase-3, nNOS protein, GSK-3β,p-GSK-3β were assessed by Western blot, and histopathological changes were assessed using Nissl stain and TUNEL stain. From P28, we used the eight-arm radial maze test and step-through test to evaluate the influence of sevoflurane exposure on the learning and memory of juvenile rats. The results showed that neonatal sevoflurane exposure induced caspase-3 activation and histopathological changes in hippocampus can be attenuated by lithium chloride. Sevoflurane increased GSK-3β activity while pretreatment of lithium decreased GSK-3β activity. Moreover, sevoflurane showed possibly slight but temporal influence on the spatial learning and the memory of juvenile rats, and chronic use of lithium chloride might have the therapeutic effect. Our current study suggests that lithium attenuates sevoflurane induced neonatal hippocampual damage by GSK-3β pathway and might improve learning and memory deficits in rats after neonatal exposure.






Similar content being viewed by others
References
Goa KL, Noble S, Spencer CM (1999) Sevoflurane in paediatric anaesthesia: a review. Arch Neurol 1(2):127–153
Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110(3):628–637. doi:10.1097/ALN.0b013e3181974fa2
Piehl E, Foley L, Barron M, D’Ardenne C, Guillod P, Wise-Faberowski L (2010) The effect of sevoflurane on neuronal degeneration and GABAA subunit composition in a develo** rat model of organotypic hippocampal slice cultures. J Neurosurg Anesthesiol 22(3):220–229. doi:10.1097/ANA.0b013e3181e16c89
Zhang X, Xue Z, Sun A (2008) Subclinical concentration of sevoflurane potentiates neuronal apoptosis in the develo** C57BL/6 mouse brain. Neurosci Lett 447(2–3):109–114. doi:10.1016/j.neulet.2008.09.083
Dong Y, Zhang G, Zhang B, Moir RD, **a W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, **e Z (2009) The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol 66(5):620–631. doi:10.1001/archneurol.2009.48
Sun L (2010) Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 105(Suppl 1):i61–i68. doi:10.1093/bja/aeq302
Feng X, Liu JJ, Zhou X, Song FH, Yang XY, Chen XS, Huang WQ, Zhou LH, Ye JH (2012) Single sevoflurane exposure decreases neuronal nitric oxide synthase levels in the hippocampus of develo** rats. Br J Anaesth 109(2):225–233. doi:10.1093/bja/aes121
Li Q, Li H, Roughton K, Wang X, Kroemer G, Blomgren K, Zhu C (2010) Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death Dis 1:e56. doi:10.1038/cddis.2010.33
Caldero J, Brunet N, Tarabal O, Piedrafita L, Hereu M, Ayala V, Esquerda JE (2010) Lithium prevents excitotoxic cell death of motoneurons in organotypic slice cultures of spinal cord. Neuroscience 165(4):1353–1369. doi:10.1016/j.neuroscience.2009.11.034
Zhong J, Yang X, Yao W, Lee W (2006) Lithium protects ethanol-induced neuronal apoptosis. Biochem Biophys Res Commun 350(4):905–910. doi:10.1016/j.bbrc.2006.09.138
Straiko MM, Young C, Cattano D, Creeley CE, Wang H, Smith DJ, Johnson SA, Li ES, Olney JW (2009) Lithium protects against anesthesia-induced developmental neuroapoptosis. Anesthesiology 110(4):862–868. doi:10.1097/ALN.0b013e31819b5eab
Chuang DM (2005) The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci 1053:195–204. doi:10.1196/annals.1344.018
Rowe MK, Chuang DM (2004) Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med 6(21):1–18. doi:10.1017/S1462399404008385
Jope RS, Yuskaitis CJ, Beurel E (2007) Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 32(4–5):577–595. doi:10.1007/s11064-006-9128-5
Zhu ZF, Wang QG, Han BJ, William CP (2010) Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull 83(5):272–277. doi:10.1016/j.brainresbull.2010.07.008
Riadh N, Allagui MS, Bourogaa E, Vincent C, Croute F, Elfeki A (2011) Neuroprotective and neurotrophic effects of long term lithium treatment in mouse brain. Biometals 24(4):747–757. doi:10.1007/s10534-011-9433-6
Feng X, Liu JJ, Zhou X, Song FH, Yang XY, Chen XS, Huang WQ, Zhou LH, Ye JH (2012) Single sevoflurane exposure decreases neuronal nitric oxide synthase levels in the hippocampus of develo** rats. Br J Anaesth. doi:10.1093/bja/aes121
He FQ, Qiu BY, Zhang XH, Li TK, **e Q, Cui DJ, Huang XL, Gan HT (2011) Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-kappaB activation in a rat model of Alzheimer’s disease induced by amyloid-beta(1-42). Brain Res 1384:89–96. doi:10.1016/j.brainres.2011.01.103
Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, **n WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG (2011) Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacol: official publication of the American College of Neuropsychopharmacology 36(5):979–992. doi:10.1038/npp.2010.236
Liu Y, Zhuang X, Gou L, Ling X, Tian X, Liu L, Zheng Y, Zhang L, Yin X (2013) Protective effects of nizofenone administration on the cognitive impairments induced by chronic restraint stress in mice. Pharmacol Biochem Behav 103(3):474–480. doi:10.1016/j.pbb.2012.09.009
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110(4):796–804. doi:10.1097/01.anes.0000344728.34332.5d
DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G (2009) A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol 21(4):286–291. doi:10.1097/ANA.0b013e3181a71f11
Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP (2009) Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 110(4):805–812. doi:10.1097/ALN.0b013e31819c7124
Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128(5):e1053–e1061. doi:10.1542/peds.2011-0351
Berns M, Zacharias R, Seeberg L, Schmidt M, Kerner T (2009) Effects of sevoflurane on primary neuronal cultures of embryonic rats. Eur J Anaesthesiol 26(7):597–602
Pan Z, Lu XF, Shao C, Zhang C, Yang J, Ma T, Zhang LC, Cao JL (2011) The effects of sevoflurane anesthesia on rat hippocampus: a genomic expression analysis. Brain Res 1381:124–133. doi:10.1016/j.brainres.2011.01.020
Wada A, Yokoo H, Yanagita T, Kobayashi H (2005) Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci 99(4):307–321
Chuang DM, Chen RW, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P (2002) Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord 4(2):129–136
Jope RS (2003) Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci 24(9):441–443. doi:10.1016/S0165-6147(03)00206-2
Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK (2000) Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75(4):1729–1734
Luo CX, Zhu XJ, Zhou QG, Wang B, Wang W, Cai HH, Sun YJ, Hu M, Jiang J, Hua Y, Han X, Zhu DY (2007) Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J Neurochem 103(5):1872–1882. doi:10.1111/j.1471-4159.2007.04915.x
Weitzdoerfer R, Hoeger H, Engidawork E, Engelmann M, Singewald N, Lubec G, Lubec B (2004) Neuronal nitric oxide synthase knock-out mice show impaired cognitive performance. Nitric Oxide 10(3):130–140. doi:10.1016/j.niox.2004.03.007S1089860304000588
Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, Toyama K, Takao K, Miyakawa T (2009) Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain 2:19. doi:10.1186/1756-6606-2-19
Liu XS, Xue QS, Zeng QW, Li QA, Liu JA, Feng XM, Yu BW (2010) Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3 beta in the hippocampus. Neurobiol Learn Mem 94(4):461–467. doi:10.1016/j.nlm.2010.08.011
Wiklund A, Granon S, Faure P, Sundman E, Changeux JP, Eriksson LI (2009) Object memory in young and aged mice after sevoflurane anaesthesia. NeuroReport 20(16):1419–1423. doi:10.1097/Wnr.0b013e328330cd2b
Acknowledgments
This work was funded by National Natural Science Foundation of China, Grant No. 81571032 and Guangdong Science Technology Planning Project, Grant No. 2013B051000045.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interests
There is no conflict of interest in this work.
Ethical approval
All applicable international, national, and Institutional Animal Care and Use Committee at Sun Yat-sen University (Guangzhou, Guangdong, China; No. [2014]A-073) guidelines for the care and use of animals were followed.
Additional information
Xue Zhou and Wen-da Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, X., da Li, W., Yuan, BL. et al. Lithium Treatment Prevents Apoptosis in Neonatal Rat Hippocampus Resulting from Sevoflurane Exposure. Neurochem Res 41, 1993–2005 (2016). https://doi.org/10.1007/s11064-016-1909-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1909-x