Abstract
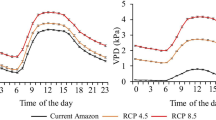
Climate change threatens many native species from the Amazon Forest. Among the endangered species is the Açaí (Euterpe oleracea), which is a species with great national and international interest, due to the nutritional benefits and medicinal properties of its fruits. However, there is still no information on the ecophysiological responses of Açaí to climate change. Thus, the objective of this work was to evaluate the effect of increased temperature and CO2 concentration change on the ecophysiology of Açaí seedlings. To do so, Açaí seedlings were subjected, for 90 days, to three different climatic scenarios: current Amazon; RCP4.5 (current average temperature in the Amazon + 2.5 °C and 538 ppm of carbon dioxide concentration i.e. CO2); and RCP8.5 (+ 4.5 °C and 936 ppm of CO2 concentration). In addition, two irrigation levels were applied within each climatic scenario: seedlings maintained at 90% (not stressed) and 40% (stressed) of the water holding capacity of the substrate. Gas exchange, water status, fluorescence parameters, enzymatic antioxidants activity and dry matter production were evaluated. High CO2 concentration enhanced Açaí gas exchange (increasing CO2 assimilation), regardless of substrate water availability and temperature. However, high temperature and high vapor-pressure deficit reduced quantum yield and increased the minimum fluorescence and enzymatic antioxidants activity. With that, Açaí seedlings did not convert the additional assimilated carbon (due to higher CO2 concentration) into biomass, showing decreased total dry mass accumulation for RCP4.5 and RCP8.5 climate scenarios. Our results indicated that the positive impacts of increased CO2 concentration to gas exchange may not offset the negative impacts of increased air temperature and VPD to Açaí growth.







Similar content being viewed by others
References
Adams WW, Muller O, Cohu CM, Demming-Adams B (2013) May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth Res 117(1–3):31–44. https://doi.org/10.1007/s11120-013-9849-7
Alvarenga CB et al (2014) Effect of the water vapor pressure deficit in the air on hydropneumatic spraying of artificial targets. Biosci J 30(1):182–193
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z. https://doi.org/10.1127/0941-2948/2013/0507
Amaral GC, Pezzopane JEM, Nóia Júnior RS, Fonseca MDS, Toledo JV, Xavier TMT, Oliveira BS, Martínez MF, Jerônimo Júnior RAC, Gonçalvez EO (2021) Ecophysiology of Pilocarpus microphyllus in response to temperature, water availability and vapour pressure deficit. Trees 35(543–555):2020. https://doi.org/10.1007/s00468-020-02055-xTrees
Amaral GC et al (2022) Pilocarpus microphyllus seedling growth threatened by climate change: an ecophysiological approach. Theor Appl Climatol 147:347–361. https://doi.org/10.1007/s00704-021-03831-6
Anderson MD, Prasad TK, Stewart CR (1995) Changes in Isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109(4):1247–1257. https://doi.org/10.1104/pp.109.4.1247
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259. https://doi.org/10.1111/j.1365-3040.2001.00668.x
Cantu-Jungles TM, Iacomini M, Cipriani TR, Cordeiro LMC (2017) Extraction and characterization of pectins from primary cell walls of edible Açaí (Euterpe oleraceae) berries, fruits of a monocotyledon palm. Carbohyd Polym 158:37–43. https://doi.org/10.1016/j.carbpol.2016.11.090
Ceulemans BR, Mousseau M (1994) Effects of elevated atmospheric CO2 on woody plants. New Phytologist Trust 127(3):425–446
Chance B, Maehly AC (1955) Assay of catalases and peroxidases: methods in enzymology. Academic Press 2:764–775
Costa GF, Marenco RA (2007) Fotossíntese, condutância estomática e potencial hídrico foliar em árvores jovens de andiroba (Carapa guianensis). Acta Amaz 37(2):229–234
de Oliveira HO, de Castro GL, Correa LO, Silvestre WV, do Nascimento SV, da Silva Valadares RB, Oliveira GC, Santos RI, Festucci-Buselli RA, Pinheiro HA (2019) Coupling physiological analysis with proteomic profile to understand the photosynthetic responses of young Euterpe oleracea palms to drought. Photosynth Res 140:189–205. https://doi.org/10.1007/s11120-018-0597-6
Del Longo OT, Gonzalez CA, Pastori GM, Trippi VS (1993) Antioxidant defences under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant Cell Physiol 34(7):1023–1028
Dikšaitytė A, Viršilė A, Žaltauskaitė J, Januškaitienė I, Juozapaitienė G (2019) Growth and photosynthetic responses in Brassica napus differ during stress and recovery periods when exposed to combined heat, drought and elevated CO2. Plant Physiol Biochem 142:59–72. https://doi.org/10.1016/j.plaphy.2019.06.026
Duursma RA, Barton CVM, Lin Y-S, Medlyn BE, Eamus D, Tissue DT, Ellsworth DS, McMurtrie RE (2014) The peaked response of transpiration rate to vapour pressure deficit in field conditions can be explained by the temperature optimum of photosynthesis. Agric for Meteorol 189–190:2–10. https://doi.org/10.1016/j.agrformet.2013.12.007
Falker (2008) Automação agrícola. Manual do medidor eletrônico de teor clorofila (ClorofiLOG/CFL 1030). Porto Alegre, p. 33
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects mechanisms and management. Sustain. Agric. Springer Netherlands, Dordrecht, pp 153–188. https://doi.org/10.1007/978-90-481-2666-8_12
Ferreira EB, Cavalcanti PP, Nogueira DA (2014) ExpDes: An R Package for ANOVA and experimental designs. Appl Math 5(19):2952–2958. https://doi.org/10.4236/am.2014.519280
Freire JC, Ribeiro MAV, Bahia VG, Lopes AS, Aquino LH (1980) Respostas do milho cultivado em casa de vegetação a níveis de água em solos da região de Lavras (MG). Rev Bras Ciênc Solo 4:5–8
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I., occurrence in higher plants. Plant Physiol 59:309–314
Guo X-Y, Zhang X-S, Huang Z-Y (2010) Drought tolerance in three hybrid poplar clones submitted to different watering regimes. J Plant Ecol 3(2):79–87. https://doi.org/10.1093/jpe/rtq007
Hartley HO (1950) The use of range in analysis of variance. Biometrika 37(3/4):271. https://doi.org/10.2307/2332380
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684. https://doi.org/10.3390/ijms14059643
Havir EA, Mchale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
He Y, Yang J, Zhu B, Zhu Z (2014) Low root zone temperature exacerbates the ion imbalance and photosynthesis inhibition and induces antioxidant responses in tomato plants under salinity. J Integr Agric 13(1):89–99. https://doi.org/10.1016/s2095-3119(13)60586-9
Hideg É, Strid A (2017) The effects of UV-B on the biochemistry and metabolism of plants. In: Jordan BR (ed) UV-B radiation and plant life: molecular biology to ecology. CABI, Wallingford, pp 90–110
IPCC (2013) Summary for policymakers. In: Climate change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.In: TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y **a, V Bex, PM Midgley, (Eds.). Cambridge University Press Cambridge, United Kingdom, New York, NY, USA 1–30
IPCC (2021) Climate Change 2021: The physical science basis. Contribution of Working Group I to the Sixth assessment report of the intergovernmental panel on climate change. In: Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.). Cambridge University Press. In Press
Jiao X-C, Song X-M, Zhang D-L, Du Q-J, Li J-M (2019) Coordination between vapor pressure deficit and CO2 on the regulation of photosynthesis and productivity in greenhouse tomato production. Sci Rep 9(1):8700. https://doi.org/10.1038/s41598-019-45232-w
Jiménez S, Dridi J, Gutiérrez D, Moret D, Irigoyen JJ, Moreno MA, Gogorcena Y (2013) Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiology 33:1061–1075. https://doi.org/10.1093/treephys/tpt074
Jordan B, Strid Å, Wargent J (2016). What role does UVB Play in determining photosynthesis? Handbook of Photosynthesis, 3rd edition, Edition: 3rd edition, Chapter: Chapter 16: 275–286. Doi:https://doi.org/10.1201/b19498-21
Kalina J, Urban O, Čajánek M, Kurasová I, Špunda V, Marek MV (2001) Different responses of norway spruce needles from shaded and exposed crown layers to the prolonged exposure to elevated CO2 studied by various chlorophyll a fluorescence techniques. Photosynthetica 39(3):369–376
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kimball BA et al (2001) Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytol 150(2):295–303
Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Experim Botany 60(10):2859–2876. https://doi.org/10.1093/jxb/erp096
Li J, Cang Z, Jiao F, Bai X, Zhang D, Zhai R (2017) Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J Saudi Soc Agric Sci 16(1):82–88. https://doi.org/10.1016/j.jssas.2015.03.001
Marenco RA, Antezana-Vera SA, Gouvêa PR, Camargo MA, Oliveira MF, Santos JK (2014) Fisiologia de espécies florestais da Amazônia: fotossíntese, respiração e relações hídricas. Rev Ceres 61:786–799. https://doi.org/10.1590/0034-737x201461000004
Marenco RA, Lopes NF (2005). Fisiologia Vegetal: Fotossíntese, respiração, relações hídricas e nutrição mineral. 451p
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: Response to high temperature stress. J Photochem Photobiol B: Biol 137:116–126
Mishra AN, Terashim, I (2003). Changes in photosystem activities during adapta-tion of Vicia faba seedlings to low, moderate and high temperature. Plant Cell Physiology, Annual Symposium JSPP, pp 27–29
Mishra AN, Srivastava A, Strasser RJ (2007). Elastic and plastic responses of Viciafaba leaves to high temperature and high light stress. In: Gordon conference on temperature stress in plants. Ventura, USA, pp 25–30
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Experim Botany 64(13):3983–3998. https://doi.org/10.1093/jxb/ert208
Neves LH, Santos RIN, Teixeira GIS, Araujo DG, Silvestre WVD, Pinheiro HA (2019) Leaf gas exchange, photochemical responses and oxidative damages in assai (Euterpe oleracea Mart) seedlings subjected to high temperature stress. Sci Horticult 257:108733. https://doi.org/10.1016/j.scienta.2019.108733
Niinemets Ü, Díaz-Espejo A, Flexas J, Galmés J, Warren CR (2009) Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. J Exp Bot 60(8):2271–2282. https://doi.org/10.1093/jxb/erp063
Nóia Júnior RS, Pezzopane JEM, Vinco JS, Xavier TMT, Cecílio RA, Pezzopane JRM (2018) Characterization of photosynthesis and transpiration in two rubber tree clones exposed to thermal stress. Brazilian J Bot 41(4):785–794. https://doi.org/10.1007/s40415-018-0495-3
Nóia Júnior RS, Amaral GC, Pezzopane JEM, Fonseca MDS, Silva APC, Xavier TMT (2019) Ecophysiological acclimatization to cyclic water stress in Eucalyptus. J Forestry Res 31(3):797–806. https://doi.org/10.1007/s11676-019-00926-9
Oliveira LC, Oliveira MSP, Davide LC, Torres GA (2016) Karyotype and genome size in Euterpe Mart (Arecaceae) species. Comparative Cytogenetics 10(1):17–25. https://doi.org/10.3897/CompCytogen.v10i1.5522
Omidi H, Shams H, Sahandi MS, Rajabian T, Miransari M (2018) Balangu (Lallemantia sp.) growth and physiology under field drought conditions affecting plant medicinal content. Plant Physiol Biochem 130:641–646. https://doi.org/10.1016/j.plaphy.2018.08.014
Peixoto PH, Cambraia J, Sant’ Anna R, Mosquim PR, Moreira MA (1999) Aluminum effects on lipid peroxidation and on the activities of enzymes of oxidative metabolism in sorghum. Rev Bras Fisiol Veg 11(3):137–143
Perdomo JA, Capó-Bauçà S, Carmo-Silva S, Galmés J (2017) Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front Plant Sci 8(13):490. https://doi.org/10.3389/fpls.2017.00490/full
Pinheiro JU, Neves JA, Cheves RE, Mendes D, Barreto NJC (2014). Avaliação de Modelos do CMIP5 que Melhor Expressam a Atuação dos Vórtices Ciclônicos em Altos Níveis (Vcans) no Nordeste Brasileiro (NEB). Revista Brasileira de Geografia Física, 7(5), (Número Especial-VIWMCRHPE):891–904
Reich PB, Sendall KM, Stefanski A, Wei X, Rich RL, Montgomery RA (2016) Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531:633–636. https://doi.org/10.1038/nature17142
Rosa BL, Souza JP, Pereira EG (2019) Increased atmospheric CO2 changes the photosynthetic responses of Acrocomia aculeata (Arecaceae) to drought. Acta Botanica Brasilica 33(3):486–497. https://doi.org/10.1590/0102-33062019abb0056
Rufino MSM, Pérez-Jiménez J, Arranz S, Alves RE, de Brito ES, Oliveira MSP, Saura-Calixto F (2011) Açaí (Euterpe oleraceae) ‘BRS Pará’: a tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Res Int 44(7):2100–2106. https://doi.org/10.1016/j.foodres.2010.09.011
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant, Cell Environ 30(9):1086–1106
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52(3–4):591–611
Silva PA, Cosme VS, Rodrigues KCB, Detmann KSC, Leão FM, Cunha RL, Buselli RAF, Damatta FM, Pinheiro HA (2017) Drought tolerance in two oil palm hybrids as related to adjustments in carbon metabolism and vegetative growth. Acta Physiol Plant 39(2):58. https://doi.org/10.1007/s11738-017-2354-4
Silvestre WVD, Pinehiro HA, Souza RORM, Palheta LF (2016) Morphological and physiological responses of Açaí seedlings subjected to different watering regimes. Revista Brasileira De Engenharia Agrícola e Ambiental 20(4):364–371. https://doi.org/10.1590/1807-1929/agriambi.v20n4p364-371
Silvestre WVD, Silva PA, Palheta LF, de Oliveira Neto CF, Souza RORM, Festucci-Buselli RA, Pinheiro HA (2017) Differential tolerance to water deficit in two Açaí (Euterpe oleracea Mart.) plant materials. Acta Physiol Plant 39(1):4. https://doi.org/10.1007/s11738-016-2301-9
Soni P, Abdin MZ (2017) Water deficit-induced oxidative stress affects artemisinin content and expression of proline metabolic genes in Artemisia annua L. FEBS Open Bio 7(3):367–381. https://doi.org/10.1002/2211-5463.12184
Šprtová M, Špunda V, Kalina J, Marek MV (2003) Photosynthetic UV-B Response of Beech (Fagus sylvatica L) Saplings. Photosynthetica 41(4):533–543. https://doi.org/10.1023/b:phot.0000027517.80915.1b
Suresh K, Nagamani C, Ramachandrudu K, Mathur RK (2010) Gas-exchange characteristics, leaf water potential and chlorophyll a fluorescence in oil palm (Elaeis guineensis Jacq.) seedlings under water stress and recovery. Photosynthetica 48(3):430–436. https://doi.org/10.1007/s11099-010-0056-x
Taiz L, Zeiger E, (2013) Fisiologia vegetal. 5. ed. Porto Alegre: Artmed, 918 p
Tang Y-Y, Yuan Y-H, Shu S, Guo S-R (2018) Regulatory mechanism of NaCl stress on photosynthesis and antioxidant capacity mediated by transglutaminase in cucumber ( Cucumis sativus L.) seedlings. Scientia Horticulturae 235:294–306
Taylor KE, Stouffer RJ, Meehl GA (2012) An overview of CMIP5 and the experiment design. Bull Am Meteorol Soc 93:485–498
Thwe AA, Kasemsap P (2014) Quantification of OJIP fluorescence transient in tomato plants Under acute ozone stress. Nat Sci 48:665–675
Tissue DT, Thomas RB, Strain BR (1993) Long-term effects of elevated CO2 and nutrients on photosynthesis and rubisco in loblolly pine seedlings. Plant Cell Environ 16(7):859–65. https://doi.org/10.1111/j.1365-3040.1993.tb00508
Tissue DT, Griffin KL, Turnbull MH, Whitehead D (2001) Canopy position and needle age affect photosynthetic response in field-grown Pinus radiata after five years of exposure to elevated carbon dioxide partial pressure. Tree Physiol 21(12–13):915–923. https://doi.org/10.1093/treephys/21.12-13.915
Urban O, Hrstka M, Holub P, Veselá B, Večeřová K, Novotná K, Grace J, Klem K (2019) Interactive effects of ultraviolet radiation and elevated CO2 concentration on photosynthetic characteristics of European beech saplings during the vegetation season. Plant Physiol Biochem 134:20–30. https://doi.org/10.1016/j.plaphy.2018.08.026
Wang X, Gao Y, Wang Q, Chen M, Ye X, Li D, Chen X, Li L, Gao D (2019) 24-Epibrassinolide-alleviated drought stress damage influences antioxidant enzymes and autophagy changes in peach (Prunus persicae L.) leaves. Plant Physiol Biochem 135:30–40. https://doi.org/10.1016/j.plaphy.2018.11.026
Way DA, Oren R, Kroner Y (2015) The space-time continuum: the effects of elevated CO2 and temperature on trees and the importance of scaling. Plant Cell Environ 38:991–1007. https://doi.org/10.1111/pce.12527
Wu YJ, Ren C, Tian Y, Zha TS, Liu P, Bai YJ, Ma JY, Lai ZR, Bourquea CP-A (2018) Photosynthetic gas-exchange and PSII photochemical acclimation to drought in a native and non-native xerophytic species (Artemisia ordosica and Salix psammophila). Ecol Ind 94:130–138
Wullschleger SD, Norby RJ, Hendrix DL (1992) Carbon exchange rates, chlorophyll content, and carbohydrate status of two forest tree species exposed to carbon dioxide enrichment. Tree Physiol 10(1):21–31. https://doi.org/10.1093/treephys/10.1.21
Yamaguchi KKL, Pereira LFR, Lamarão CV, Lima ES (2015) Amazon acai: chemistry and biological activities: a review. Food Chem 179:137–151
Zha T-S, Wu YJ, Jia X, Zhang MY, Bai YJ, Liu P, Ma JY, Bourque CP-A, Peltola H (2017) Diurnal response of effective quantum yield of PSII photochemistry to irradiance as an indicator of photosynthetic acclimation to stressed environments revealed in a xerophytic species. Ecol Ind 74:191–197. https://doi.org/10.1016/j.ecolind.2016.11.027
Acknowledgements
This work is supported by FAPES (Research Support Foundation of Espírito Santo) with research funding and doctoral scholarships to the first author (Edital PROCAP 2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amaral, G.C., Pezzopane, J.E.M., de Souza Nóia Júnior, R. et al. Climate change and the growth of Amazonian species seedlings: an ecophysiological approach to Euterpe oleracea. New Forests 54, 269–287 (2023). https://doi.org/10.1007/s11056-022-09921-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-022-09921-1