Abstract
Magnetic composite adsorbents using iron oxide nanoparticles have been produced in the past for glyphosate removal; however, the effect of the magnetic iron oxide content in the chitosan/calcium alginate hydrogel matrix on glyphosate removal efficiency has not been investigated. In this study, magnetic chitosan/calcium alginate hydrogel (MCAH) beads were synthesized according to our previous study with the same methodology with a magnetic iron oxide nanoparticle (MIONP) content of 33%, 50%, and 60% (w/w). Iron content was analyzed by characterization study. The increasing iron weight percentage was detected at the magnetic beads with the FESEM-EDX analysis. The magnetism of the MCAH beads increased with the higher MIONP content according to VSM analysis. The surface area and porosity of MCAH beads decreased with MIONP content increase according to the BET analysis. The results showed that the higher MIONP content increases the reaction rate by 13 percent but does not change the resultant removal efficiency significantly.
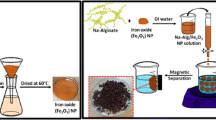
Graphical Abstract







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (D. Aksu Demirezen) upon reasonable request.
References
**a C, Geng H, Li X, Zhang Y, Wang F, Tang X, Blake R, Li H, Chang S (2019) Mechanism of methylphosphonic acid photo-degradation based on phosphate oxygen isotopes and density functional theory. RSC Adv 9:31325–31332
Xue L, Hao L, Ding H, Liu R, Zhao D, Fu J, Zhang M (2021) Complete and rapid degradation of glyphosate with Fe3Ce1Ox catalyst for peroxymonosulfate activation at room temperature. Environmental Res 201:111618
Sereshti H, Duman O, Tunç S, Nouri N, Khorram P (2020) Nanosorbent-based solid phase microextraction techniques for the monitoring of emerging organic contaminants in water and wastewater samples. Microchim Acta 187:541
Kaur R, Hasan A, Iqbal N, Alam S, Saini MK, Raza SK (2014) Synthesis and surface engineering of magnetic nanoparticles for environmental cleanup and pesticide residue analysis: A review. J Seperation Sci 37(14):1805–1825
Wu W, Wu Z, Yu T, Jiang C, Kim W-S (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mat 16:023501
Shabnoor N, Bindu AH, Patil AG, Aishwarya S, More SS, Khan K, Padyana S, Madhavi J, Yadav AN, Ravish H, Manjunath P, Sahu B, Raghu A, Zameer F (2023) Chapter 8 - Peptide and protein-based hydrogels for the encapsulation of bioactive compounds and tissue engineering applications," in Protein-Based Biopolymers, Woodhead Publishing. 213–238,.
Divyashri G, Badhe RV, Sadanandan B, Vijayalakshmi V, Kumari M, Ashrit P, Bijukumar D, Mathew MT, Shetty K, Raghu AV (2022) Applications of hydrogel-based delivery systems in wound care and treatment: An up-to-date review. Polym Adv Technol 33(7):2025–2043
Allouss D, Makhado E, Zahouily M (2022) Recent Progress in Polysaccharide-Based Hydrogel Beads as Adsorbent for Water Pollution Remediation. Functional Polymer Nanocomposites for Wastewater Treatment 323:55–88
Qu B, Luo Y (2020) Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors – A review. Int J Biol Macromol 152:437–448
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 4:441–456
Pujari SP, Scheres L, Marcelis AT, Zuilhof H (2014) Covalent surface modification of oxide surfaces. Angew Chem Int Ed 53(25):6322–6356
Zeininger L, Portilla L, Halik M, Hirsch A (2016) Quantitative Determination and Comparison of the Surface Binding of Phosphonic Acid, Carboxylic Acid, and Catechol Ligands on TiO2 Nanoparticles. Chem A Europ J 22(38):13506–13512
Yu P, Lia X, Zhang X, Zhou H, Xu Y, Sun Y, Zheng H (2021) Insights into the glyphosate removal efficiency by using magnetic powder. Separation Purification Technol 254:117662
Brice˜no S, Reinoso C (2022) CoFe2O4-chitosan-graphene nanocomposite for glyphosate removal,". Environmental Res 212:113470
Li C, Li Y, Li Q, Duan J, Hou J, Hou Q, Ai S, Li H, Yang Y (2021) Regenerable magnetic aminated lignin/Fe3O4/La(OH)3 adsorbents for the effective removal of phosphate and glyphosate. Sci Total Environ 788:147812
Soares SF, Amorim CO, Amaral JS, Trindade T, Daniel-da-Silva AL (2021) On the efficient removal, regeneration and reuse of quaternary chitosan magnetite nanosorbents for glyphosate herbicide in water. J Environ Chem Eng 9:105189
Esmaeilian A, Dionysiou DD, O’Shea KE (2022) Incorporating simultaneous effect of initial concentration and sorbent dose into removal prediction model using glyphosate experimental data and theoretical analysis. Chem Eng J 445:136667
Demirezen DA, Yılmaz DD, Yıldız YŞ (2023) Magnetic chitosan/calcium alginate double-network hydrogel beads: Preparation, adsorption of anionic and cationic surfactants, and reuse in the removal of methylene blue. Int J Biol Macromol 239:124311
Bhaskara BL, Nagaraja P (2006) Direct Sensitive Spectrophotometric Determination of Glyphosate by Using Ninhydrin as a Chromogenic Reagent in Formulations and Environmental Water Samples. Helvetica Chimica Acta 89(11):2686–2693
Almessiere M, Slimani Y, Korkmaz A, Guner S, Sertkol M, Shirsath SE, Baykal A (2019) Structural, optical and magnetic properties of Tm3+ substituted cobalt spinel ferrites synthesized via sonochemical approach. Ultrason Sonochem 54:1–10
Faham M, Shokrollahi H, Yousefi G, Abbasi S (2014) PEG decorated glycine capped Mn-Ferrite nanoparticles synthesized by co-precipitation method for biomedical application. Adv Mat Res 829:274–278
Cui Z, **ang Y, Si J, Yang M, Zhang Q, Zhang T (2008) Ionic interactions between sulfuric acid and chitosan membranes. Carbohyd Polym 73(1):111–116
Baran EJ (2008) Spectroscopic investigation of the VO2+/chitosan interaction. Carbohyd Polym 74(3):704–706
Ghosh A, Ali MA (2012) Studies on physicochemical characteristics of chitosan derivatives with dicarboxylic acids. J Mater Sci 47(3):1196–1204
Jiang Z, Dou G (2020) Preparation and Characterization of Chitosan Grafting Hydrogel for Mine-Fire Fighting. ACS Omega 5:2303–2309
Chen A, Chen S (2009) Biosorption of azo dyes from aqueous solution by glutaraldehyde-crosslinked chitosans. J Hazard Mater 172(2–3):1111–1121
Fauzi NIM, Fen YW, Omar NAS, Saleviter S, Daniyal WMEMM, Hashim HS, Nasrullah M (2020) Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation. Polymers 12:1497
Duan GW, Zhang **, Li Yang, Yuan Ming Xu, Yin Fang, Yun Zhi Fu (2017) The preparation of Fe3O4/molecular-imprinted nanocomposite and the application on the recognition and separation of glyphosate. Inorg Nano-Metal Chem 47(4):481–487
Shooto ND (2020) Removal of toxic hexavalent chromium (Cr(VI)) and divalent lead (Pb(II)) ions from aqueous solution by modified rhizomes of Acorus calamus. Surfaces and Interfaces 20:100624
Mitra M, Raghavaiah P, Ghosh R (2015) A mononuclear cobalt(iii) complex and its catecholase activity. New J Chem 39:200–205
Divyashri G, Murthy TPK, Ragavan KV, Sudha LS, Nishka S, Himanshi G, Misriya N, Sharada B, Venkataramanaiah RA (2023) Valorization of coffee bean processing waste for the sustainable extraction of biologically active pectin. Heliyon 9:9
Ramrakhiani L, Ghosh S, Mandal AK, Majumdar S (2019) Utilization of multi-metal laden spent biosorbent for removal of glyphosate herbicide from aqueous solution and its mechanism elucidation. Chem Eng J 361:1063–1077
Liu B, Dong L, Yu Q, Li X, Wu F, Tan Z, Luo S (2016) Thermodynamic Study on the Protonation Reactions of Glyphosate in Aqueous Solution: Potentiometry, Calorimetry and NMR spectroscopy. J Phys Chem B 120(9):2132–2137
Sultan M, Tahir H, Ahmed K, Jahanzeb Q (2011) The kinetics study of the reduction of Fast Green dye with cetylpyridinum chloride as cationic surfactant. Front Chem China 6:105–112
Wu J, Wang Y, Wu Z, Gao Y, Li X (2020) Adsorption properties and mechanism of sepiolite modified by anionic and cationic surfactants on oxytetracycline from aqueous solutions. Sci Total Environ 708:134409
Jiang X, Ouyang Z, Zhang Z, Yang C, Li X, Dang Z, Wu P (2018) Mechanism of glyphosate removal by biochar supported nano-zero-valent. Colloids Surf A 547:64–72
Funding
This research study was supported by the Erciyes University Scientific Research Project Coordination Department with project number FBA-2022–11387.
Author information
Authors and Affiliations
Contributions
Derya Aksu Demirezen: methodology, investigation, data curation, writing-review and editing. Dilek Demirezen Yılmaz: resources. Yalçın Şevki Yıldız: funding acquisition, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aksu Demirezen, D., Demirezen Yılmaz, D. & Yıldız, Y.Ş. Determination of the effect of iron oxide nanoparticle content in magnetic chitosan/calcium alginate hydrogel matrix on the removal of glyphosate from water. J Nanopart Res 26, 15 (2024). https://doi.org/10.1007/s11051-024-05928-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-05928-1