Abstract
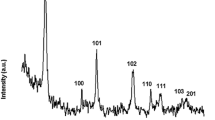
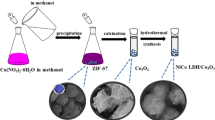
In this work, nickel–cobalt double hydroxide nanosheets with high rate capability are prepared by a facile epoxide precipitation route. The synthetic procedure includes an oxidization step using ammonium persulfate as oxidant and a precipitation step using propylene oxide as precipitation agent. As shown in the results of electrochemical characterization, high specific capacitance of 2548 F g−1 for this material can be obtained at current density of 0.9 A g−1 in aqueous solution of 3 mol L−1 KOH. It is surprising to notice that the capacitance of material still remains 1587 F g−1 at high current density of 35.7 A g−1. These results demonstrate that the as-prepared nickel–cobalt double hydroxide nanosheets are promising electrode material for supercapacitor application as a primary power source.






Similar content being viewed by others
References
Begum S, Muralidharan V, Ahmedbasha C (2009) The influences of some additives on electrochemical behaviour of nickel electrodes. Int J Hydrog Energy 34:1548–1555
Bing L, Yuan HT, Zhang YS, Zhou ZX, Song DY (1999) Cyclic voltammetric studies of stabilized α-nickel hydroxide electrode. J Power Sources 79:277–280
Chen X, Chen XH, Zhang FQ, Yang Z, Huang SM (2013) One-pot hydrothermal synthesis of reduced graphene oxide/carbon nanotube/α-Ni(OH)2 composites for high performance electrochemical supercapacitor. J Power Sources 243:555–561
Chen H, Hu LF, Chen M, Yan L, Wu LM (2014) Nickel–Cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv Funct Mater 24:934–942
Cheng YW, Zhang HB, Varanasi CV, Liu J (2013) Improving the performance of cobalt–nickel hydroxide- based self-supporting electrodes for supercapacitors using accumulative approaches†. Energy Environ Sci 6:3314–3321
Cui HT, Liu Y, Ren WZ (2013a) Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol–gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv Powder Technol 24:93–97
Cui HT, Zhao YN, Ren WZ, Wang MM, Liu Y (2013b) Large scale selective synthesis of & α-Co(OH)2 and b-Co(OH)2 nanosheets through a fluoride ions mediated phase transformation process. J Alloys Compd 562:33–37
Deki S, Hosokawa A (2013) α-Ni(OH)2 thin films fabricated by liquid phase deposition method. Thin Solid Films 517:1546–1554
Gupta V, Gupta S, Miura N (2008) Potentiostatically deposited nanostructured Co x Ni1−x layered double hydroxides as electrode materials for redox-supercapacitors. J Power Sources 175:680–685
Hu ZA, **e YL, Wang YX, Wu HY, Yang YY, Zhang ZY (2009a) Electrochimica acta synthesis and electrochemical characterization of mesoporous Co x Ni1−x layered double hydroxides as electrode materials for supercapacitors. Electrochim Acta 54:2737–2741
Hu ZA, **e YL, Wang YX, **e LJ, Fu GR, ** XQ, Zhang ZY, Yang YY, Wu HY (2009b) Synthesis of α-cobalt hydroxides with different intercalated anions and effects of intercalated anions on their morphology, basal plane spacing, and capacitive property. J Phys Chem C 113:12502–12508
Kong XH, Liu XB, He YD, Zhang DS, Wang XF, Li YD (2007) Hydrothermal synthesis of β-nickel hydroxide microspheres with flakelike nanostructures and their electrochemical properties. Mater Chem Phys 106:375–378
Kong LB, Lang JW, Liu M, Luo YC, Kang L (2009) Facile approach to prepare loose-packed cobalt hydroxide nano-flakes materials for electrochemical capacitors. J Power Sources 194:1194–1201
Lee JW, Ahn T, Soundararajan D, Ko JM, Kim JD (2011) Non-aqueous approach to the preparation of reduced graphene oxide/α-Ni(OH)2 hybrid composites and their high capacitance behavior. Chem Commun 47:6305–6307
Lei LX, Hu M, Gao XR, Sun YM (2008) The effect of the interlayer anions on the electrochemical performance of layered double hydroxide electrode materials. Electrochim Acta 54:671–676
Li YW, Yao JH, Liu CJ, Zhao WM, Deng WX, Zhong SK (2010) Effect of interlayer anions on the electrochemical performance of Al-substituted α-type nickel hydroxide electrodes. Int J Hydrog Energy 35:2539–2545
Li JX, Yang M, Zhou Z (2012) Nanoscale Ni(OH)2–Co(OH)2 composites as pseudocapacitor materials. Nanoscale 4:4498–4503
Liang JB, Ma RZ, Iyi N, Ebina Y, Takada K, Sasaki T (2010) Topochemical synthesis, anion exchange, and exfoliation of Co–Ni layered double hydroxides: a route to positively charged Co–Ni hydroxide nanosheets with tunable composition. Chem Mater 22:371–378
Liu XH, Ma RZ, Bando Y, Sasaki T (2012) A general strategy to layered transition-metal hydroxide nanocones: tuning the composition for high electrochemical performance. Adv Mater 24:2148–2153
Mondal C, Ganguly M, Manna PK, Yusuf SM, Pal T (2013) Fabrication of porous β–Co(OH)2 architecture at room temperature: a high performance supercapacitor. Langmuir 29:9179
Oshitani M, Yufu H, Takashima K, Tsuji S, Matsumaru Y (1989) Development of a pasted nickel electrode with high active material utilization. J Electrochem Soc 136:1590–1593
Pan T, Wang JM, Zhao YL, Chen H, **ao HM, Zhang JQ (2003) Al-stabilized α-nickel hydroxide prepared by electrochemical impregnation. Mater Chem Phys 78:711–718
Park JH, Kim S, Park OO, Ko JM (2005) Improved asymmetric electrochemical capacitor using Zn–Co co-doped Ni(OH)2 positive electrode material. Appl Phys A 82:593–597
Pu J, Tong Y, Wang SB, Sheng EH, Wang ZH (2014) Nickelecobalt hydroxide nanosheets arrays on Ni foam for pseudocapacitor applications. J Power Sources 250:250–256
Rajeswari J, Kishore PS, Viswanathan B, Varadarajan TK (2009) One-dimensional MoO2 nanorods for supercapacitor applications. Electrochem Commun 11:572–575
Vidotti M, Silva MR, Salvador RP, Torresi SLC, Dall’Antonia LH (2008) Electrocatalytic oxidation of urea by nanostructured nickel/cobalt hydroxide electrodes. Electrochim Acta 53:4030–4034
Wang GP, Zhang L, Kim J, Zhang JJ (2012) Nickel and cobalt oxide composite as a possible electrode material for electrochemical supercapacitors. J Power Sources 217:554–561
Wang MM, Ren WZ, Zhao YN (2013) One-pot synthesis of powder-form & α-Ni(OH)2 monolayer nanosheets with high electrochemical performance. J Nanopart Res 15:1849
Wu JB, Tu JP, Wang XL, Zhang WK (2007) Synthesis of nanoscale CoO particles and their effect on the positive electrodes of nickel–metal hydride batteries. Int J Hydrog Energy 32:606–610
**e LJ, Hu ZA, Lv CX, Sun GH, Wang JL, Li YQ, He HW, Wang J, Li KX (2012) Co x Ni1−x double hydroxide nanoparticles with ultrahigh specific capacitances as supercapacitor electrode materials. Electrochim Acta 78:205–211
Xu J, Gao L, Cao JY, Wang WC, Chen ZD (2010) Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim Acta 56:732–736
Xu J, Dong YZ, Cao JY, Guo B, Wang WC, Chen ZD (2013) Electrochimica acta microwave-incorporated hydrothermal synthesis of urchin-like Ni(OH)2–Co(OH)2 hollow microspheres and their supercapacitor applications. Electrochim Acta 114:76–82
Yan T, Li ZJ, Ruiyi Li, Qi N, Hui K, Niu YL, Liu JK (2012) Nickel–cobalt double hydroxides microspheres with hollow interior and hedgehog-like exterior structures for supercapacitors. J Mater Chem 22:23587–23592
Yang GW, Xu CL, Li HL (2008) Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem Commun 6537–6539. doi:10.1039/b815647f
Zhao Y (2004) Al-substituted α-nickel hydroxide prepared by homogeneous precipitation method with urea. Int J Hydrog Energy 29:889–896
Zhong JH, Wang AL, Li GR, Wang JW, Ou YN, Tong YX (2012) Co3O4/Ni(OH)2 composite mesoporous nanosheet networks as a promising electrode for supercapacitor applications. J Mater Chem 22:5656
Zhuo LH, Ge JC (2009) Solvothermal synthesis of CoO, Co3O4, Ni(OH)2 and Mg(OH)2 nanotubes. Cryst Growth Des 9:5–10
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, M., Xue, J., Zhang, F. et al. Facile synthesis of nickel–cobalt double hydroxide nanosheets with high rate capability for application in supercapacitor. J Nanopart Res 17, 106 (2015). https://doi.org/10.1007/s11051-015-2918-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-2918-4