Abstract
Background
The presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and its receptor in various testicular cells and spermatozoa suggests a potential role in enhancing spermatogonial and postmeiotic cell development. Moreover, GM-CSF activates the pivotal pathways implicated in sperm motility regulation and glucose metabolism. However, the impact of GM-CSF on testicular biopsies from patients with obstructive azoospermia (OA) remains unexplored. Therefore, this study aimed to investigate the in vitro effects of GM-CSF on the expression of genes related to glucose transporters and signaling pathways, sperm motility, and viability in testicular biopsies.
Methods and results
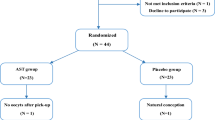
Following testicular sperm extraction from 20 patients diagnosed with OA, each sample was divided into two parts: the experimental samples were incubated with medium containing 2 ng/ml GM-CSF at 37 °C for 60 min, and the control samples were incubated with medium without GM-CSF. Subsequently, the oocytes retrieved from the partner were injected with sperm from the treatment and control groups. The sperm parameters (motility and viability), the expression levels of sperm motility-related genes (PIK3R1, PIK3CA, and AKT1), and the expression levels of sperm energy metabolism-related genes (GLUT1, GLUT3, and GLUT14) were assessed. Furthermore, the fertilization and day 3 embryo development rate and embryo quality were evaluated. Compared with those in the nontreated group, the motility parameters and the mRNA expression levels of PIK3R1, AKT1, and GLUT3 in testicular sperm supplemented with GM-CSF were significantly greater (p < 0.05). However, no significant differences in the mRNA expression of PIK3CA, GLUT1, or GLUT14 were detected. According to the ICSI results, compared with the control group, the GM-CSF treatment group exhibited significantly greater fertilization rates (p = 0.027), Day 3 embryo development rate (p = 0.001), and proportions of good-quality embryos (p = 0.002).
Conclusions
GM-CSF increased the expression of genes related to motility and the energy metabolism pathway and effectively promoted the motility of testis-extracted spermatozoa, consequently yielding positive clinical outcomes.




Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- ICSI:
-
Intracytoplasmic sperm injection
- ART:
-
Assisted reproductive technology
- TESE:
-
Testicular sperm extraction
- PTF:
-
Pentoxifylline
- GLUTs:
-
Glucose transporters
- GLUT1:
-
Glucose transporter 1
- PI3K/AKT:
-
Phosphoinositide-3-kinase/protein kinase B
- OAT:
-
Oligoasthenoteratospermia
- GnRH:
-
Gonadotropin-releasing hormone
- hCG:
-
Human chorionic gonadotrophin
References
Moein MR et al (2015) Evaluation of sperm retrieval rate with bilateral testicular sperm extraction in infertile patients with azoospermia. Iran J Reprod Med 13(11):711
Schill T et al (2003) Clinical and endocrine follow-up of patients after testicular sperm extraction. Fertil Steril 79(2):281–286
Nagy Z et al (1995) Andrology: the result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Hum Reprod 10(5):1123–1129
Ortega C et al (2011) Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update 17(5):684–692
Stalf T et al (2005) Influence of motility and vitality in intracytoplasmic sperm injection with ejaculated and testicular sperm. Andrologia 37(4):125–130
Angelopoulos T et al (1999) Enhancement or initiation of testicular sperm motility by in vitro culture of testicular tissue. Fertil Steril 71(2):240–243
Kovačič B, Vlaisavljević V, Reljič M (2006) Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. Journal of andrology 27(1):45–52
Aliabadi E et al (2012) Effects of l-carnitine and l-acetyl-carnitine on testicular sperm motility and chromatin quality. Iran J Reprod Med 10(2):77
McLay RN, Banks WA, Kastin AJ (1997) Granulocyte macrophage-colony stimulating factor crosses the blood-testis barrier in mice. Biol Reprod 57(4):822–826
Ziebe S et al (2013) A randomized clinical trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) in embryo culture medium for in vitro fertilization. Fertil Steril 99(6):1600.e2-1609.e2
Zhao JM et al (2003) Clinical pathology and pathogenesis of severe acute respiratory syndrome. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 17(3):217–221
Vilanova LT et al (2003) Expression of granulocyte–macrophage colony stimulating factor (GM-CSF) in male germ cells: GM-CSF enhances sperm motility. Theriogenology 60(6):1083–1095
Padilla L et al (2020) Granulocyte-macrophage colony stimulating factor (GM-CSF) is fully expressed in the genital tract, seminal plasma and spermatozoa of male pigs. Sci Rep 10(1):1–12
Rodríguez-Gil J et al (2007) Expression of the GM-CSF receptor in ovine spermatozoa: GM-CSF effect on sperm viability and motility of sperm subpopulations after the freezing–thawing process. Theriogenology 67(8):1359–1370
Jorban A, Lunenfeld E, Huleihel M (2023) Granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced maturation of spermatogonial cells from prepubertal mice in vitro is enhanced by testosterone. Eur Cytokine Netw 34(4):54–62
Niemi M, Sharpe R, Brown W (1986) Macrophages in the interstitial tissue of the rat testis. Cell Tissue Res 243(2):337–344
Kern S et al (1995) Cytokine secretion by macrophages in the rat testis. Biol Reprod 53(6):1407–1416
Rubenstein M et al (1991) GM-CSF restoration of a differentiated (growth factor-regulated) phenotype in an anaplastic tumor. Urol Res 19(5):309–312
Ota K et al (2013) Expression of a2 vacuolar ATPase in spermatozoa is associated with semen quality and chemokine-cytokine profiles in infertile men. PLoS ONE 8(7):e70470
Hosseini E et al (2024) Granulocyte-macrophage colony-stimulating factor cytokine addition after the freeze-thawing process improves human sperm motility and vitality in asthenoteratozoospermia patients. Biopreserv Biobank 22(1):38–45
Datta S, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13:2905–2927
Zambrano A et al (2010) Cytokine stimulation promotes increased glucose uptake via translocation at the plasma membrane of GLUT1 in HEK293 cells. J Cell Biochem 110(6):1471–1480
Sagare-Patil V et al (2013) Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction. Mol Cell Endocrinol 374(1–2):82–91
Dias TR et al (2014) Sperm glucose transport and metabolism in diabetic individuals. Mol Cell Endocrinol 396(1–2):37–45
Navale AM, Paranjape AN (2016) Glucose transporters: physiological and pathological roles. Biophys Rev 8(1):5–9
Alves MG et al (2013) Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers 1(2):e23992
Ahmed AA et al (2020) Gum Arabic improves the reproductive capacity through upregulation of testicular glucose transporters (GLUTs) mRNA expression in Alloxan induced diabetic rat. Bioact Carbohydr Diet Fibre 22:100218
Kishimoto A et al (2015) Immunohistochemical localization of GLUT3, MCT1, and MCT2 in the testes of mice and rats: the use of different energy sources in spermatogenesis. Biomed Res 36(4):225–234
Tanhaye Kalate Sabz F et al (2022) GM–CSF (granulocyte-macrophage colony-stimulating factor) treatment improves sperm parameters in men with oligoasthenoteratospermia via PI3K/AKT pathway. Andrologia 54(7):14427
Nikmard F et al (2016) A comparative study on the results of agonist and antagonist protocols based on serum AMH levels in patients undergoing intracytoplasmic sperm injection. Int J Reprod BioMed 14(12):769
The Vienna consensus (2017) report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online 35(5):494–510
The Istanbul consensus workshop on embryo assessment (2011) proceedings of an expert meeting. Hum Reprod 26(6):1270–1283
Edition F (2010) Examination and processing of human semen. World Health [Internet]
Lacham-Kaplan O, Trounson A (1993) The effects of the sperm motility activators 2-deoxyadenosine and pentoxifylline used for sperm micro-injection on mouse and human embryo development. Hum Reprod 8(6):945–952
Shi J-Z et al (2010) Expressions of sperm-specific genes in carnitine-cultured testis sperm of obstructive azoospermia patients. Natl J Androl 16(6):504–509
Robertson SA et al (2001) Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod 64(4):1206–1215
Peralta O et al (2016) Tissue localization of GM-CSF receptor in bovine ovarian follicles and its role on glucose uptake by mural granulosa cells. Anim Reprod Sci 170:157–169
Angulo C et al (1998) Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem 71(2):189–203
Burant C, Davidson N (1994) GLUT3 glucose transporter isoform in rat testis: localization, effect of diabetes mellitus, and comparison to human testis. Am J Physiol-Regul Integr Comp Physiol 267(6):R1488–R1495
Costa J et al (2023) Mitochondria quality control and male fertility. Biology. https://doi.org/10.3390/biology12060827
Barbagallo F et al (2020) Evaluation of sperm mitochondrial function: a key organelle for sperm motility. J Clin Med. https://doi.org/10.3390/jcm9020363
Wessendarp M et al (2022) Role of GM-CSF in regulating metabolism and mitochondrial functions critical to macrophage proliferation. Mitochondrion 62:85–101
Wang Y-Y et al (2021) Protein kinases regulate hyperactivated motility of human sperm. Chin Med J 134(20):2412–2414
Zhang J et al (2017) Leucine mediates autophagosome-lysosome fusion and improves sperm motility by activating the PI3K/Akt pathway. Oncotarget 8(67):111807
Pujianto DA, Curry BJ, Aitken RJ (2010) Prolactin exerts a prosurvival effect on human spermatozoa via mechanisms that involve the stimulation of Akt phosphorylation and suppression of caspase activation and capacitation. Endocrinology 151(3):1269–1279
Koppers AJ et al (2011) Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J 436(3):687–698
Aitken RJ et al (2021) Evidence that extrapancreatic insulin production is involved in the mediation of sperm survival. Mol Cell Endocrinol 526:111193
Peña FJ et al (2022) An integrated overview on the regulation of sperm metabolism (glycolysis-Krebs cycle-oxidative phosphorylation). Anim Reprod Sci 246:106805
Imoedemhe DA et al (1992) The effect of caffeine on the ability of spermatozoa to fertilize mature human oocytes. J Assist Reprod Genet 9(2):155–160
Takeda N et al (2009) Clinical outcome of sorting and recovery of ultrasmall amounts of live sperm stimulated with pentoxifylline followed by intracytoplasmic sperm injection. J Mamm Ova Res 26(2):79–85
Acknowledgements
The authors would like to thank the Clinical Research Development Unit of Ayatollah Mousavi Hospital, Zanjan University of Medical Sciences, Zanjan, Iran for their cooperation and assistance throughout the period of study.
Funding
This study was financially supported by a grant from the Zanjan University of Medical Sciences (Grant number: A-11-1314-1).
Author information
Authors and Affiliations
Contributions
FTKS: Original draft preparation. EH., FSA., ZZ., and MM: Conceptualization and Methodology. EH., and FSA: Supervision. MA., and FM: patient recruitment. FTKS., and RK: Performing laboratory work, collecting the data and analysis. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The experimental protocol was approved by the ethical committee of Zanjan University of Medical Sciences, Zanjan, Iran, (IR.ZUMS.REC.1398.357). All participants signed informed consent to participate.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanhaye Kalate Sabz, F., Hosseini, E., Amjadi, F.S. et al. In vitro effect of granulocyte–macrophage colony-stimulating factor (GM-CSF) on the expression of genes related to sperm motility and energy metabolism and intracytoplasmic sperm injection outcomes in obstructive azoospermic patients. Mol Biol Rep 51, 727 (2024). https://doi.org/10.1007/s11033-024-09676-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09676-2