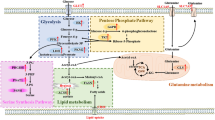
Abstract
Breast cancer is a leading cause of mortality and the most prevalent form of malignant tumor among women worldwide. Breast cancer cells exhibit an elevated glycolysis and altered glucose metabolism. Moreover, these cells display abnormal glycosylation patterns, influencing invasion, proliferation, metastasis, and drug resistance. Consequently, targeting glycolysis and mitigating abnormal glycosylation represent key therapeutic strategies for breast cancer. This review underscores the importance of protein glycosylation and glucose metabolism alterations in breast cancer. The current research efforts in develo** effective interventions targeting glycolysis and glycosylation are further discussed.


Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL et al (2020) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Chhikara BS, Parang K (2023) Global Cancer statistics 2022: the trends Projection Analysis. Chem Biol Lett 10:451
Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Polyak K (2007) Breast cancer: origins and evolution. J Clin Invest 117:3155–3163
Kleibl Z, Kristensen VN (2016) Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast 20:136–144
Scott DA, Drake RR (2019) Glycosylation and its implications in breast cancer. Expert Rev 16:665–680
Ibrahim E, Al-Gahmi AM, Zeenelin AA et al (2009) Luminal A breast cancer subtypes: a matched case-control study using estrogen receptor, progesterone receptor, and HER-2 as surrogate markers. Med Oncol 26:372–378
Apostolou P, Fostira F (2013) Hereditary breast cancer: the era of new susceptibility genes. Biomed Res Int 2013: 747318
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360:790
Fragomeni SM, Sciallis A, Jeruss JS (2018) Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am 27:95–120
Szymiczek A, Lone A, Akbari MR (2021) Molecular intrinsic versus clinical subty** in breast cancer: a comprehensive review. Clin Genet 99:613–637
Seyfried TN, Arismendi-Morillo G, Mukherjee P, Chinopoulos C (2020) On the origin of ATP synthesis in cancer. iScience 23:101761
Reily C, Stewart TJ, Renfrow MB, Novak J (2019) Glycosylation in health and disease. Nat Rev Nephrol 15:346–366
Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291:2376–2378
Slawson C, Copeland RJ, Hart GW (2010) O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 35:547–555
Hay N (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 16:635–649
Li Z, Zhang H (2016) Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci 73:377–392
Zhang X, **ang J (2019) Remodeling the Microenvironment before occurrence and metastasis of Cancer. Int J Biol Sci 15:105–113
Martínez-Reyes I, Chanel NS (2021) Cancer metabolism: looking forward. Nat Rev Cancer 21:669–680
Cairns RA, HARRIS IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95
Dias AS, Almeida CR, Helguero LA, Duarte IF (2019) Metabolic crosstalk in the breast cancer microenvironment. Eur J Cancer 121:154–171
Gill KS, Fernandes P, O’Donovan TR et al (2016) Glycolysis inhibition as a cancer treatment and its role in an anti-tumour immune response. Biochim Biophys Acta 1866:87–105
Warburg O (1956) On the origin of cancer cells. Science 3191:309–314
Wei J, Huang K, Chen Z et al (2020) Characterization of Glycolysis-Associated molecules in the Tumor Microenvironment revealed by Pan-cancer tissues and Lung Cancer single cell data. Cancers (Basel) 12:1788
Warburg O (1928) The chemical constitution of respiratory ferment. Science 68:437–443
Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270
Ganapathy-Kanniappan S, Geschwind JF (2013) Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer 12:152
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Bononi G, Masoni S, Di Bussolo V et al (2022) Historical perspective of tumor glycolysis: a century with Otto Warburg. Semin Cancer Biol 86:325–333
Ramos-Martinez JI (2017) The regulation of the pentose phosphate pathway:remember Krebs. Arch Biochem Biophys 614:50–52
Patra KC, Hay N (2014) The pentose phosphate pathway and cancer. Trends Biochem Sci 39:347–354
Shin E, Koo JS (2021) Glucose metabolism and glucose transporters in breast Cancer. Front Cell Dev Biol 9:728759
Wellen KE, Lu C, Mancuso A et al (2010) The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev 24:2784–2799
Wright EM, Loo DD, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91(2):733–794
Tsunokake S, Iwabuchi E, Miki Y et al (2023) SGLT1 as an adverse prognostic factor in invasive ductal carcinoma of the breast. Breast Cancer Res Treat 201:499–513
Liu H, Ertay A, Peng P et al (2019) SGLT1 is required for the survival of triple-negative breast cancer cells via potentiation of EGFR activity. Mol Oncol 13:1874–1886
Wang J, Ji H, Niu X et al (2020) Sodium-Dependent Glucose Transporter 1 (SGLT1) Stabled by HER2 Promotes Breast Cancer Cell Proliferation by Activation of the PI3K/Akt/mTOR Signaling Pathway in HER2 + Breast Cancer. Dis Markers 2020: 6103542
Shibazaki T, Tomae M, Ishikawa-Takemura Y et al (2012) KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 342:288–296
Fukudo S, Endo Y, Hongo M et al (2018) Safety and efficacy of the sodium-glucose cotransporter 1 inhibitor mizagliflozin for functional constipation: a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Gastroenterol Hepatol 3:603–613
Zhou J, Zhu J, Yu SJ et al (2020) Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother 132:110821
Komatsu S, Nomiyama T, Numata T et al (2020) SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J 67:99–106
Mueckler M, Thorens B (2013) The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34:121–138
Wang Z, Zhang L, Zhang D et al (2015) Glycolysis inhibitor 2-deoxy-dglucose suppresses carcinogen-induced rat hepatocarcinogenesis by restricting cancer cell metabolism. Mol Med Rep 11:1917–1924
Kuntz S, Mazerbourg S, Boisbrun M et al (2014) Energy restriction mimetic agents to target cancer cells: comparison between 2-deoxyglucose and thiazolidinediones. Biochem Pharmacol 92:102–111
Tagg SL, Foster PA, Leese MP et al (2008) 2-Methoxyoestradiol-3,17-O,O-bis-sulphamate and 2-deoxy-D-glucose in combination: a potential treatment for breast and prostate cancer. Br J Cancer 99:1842–1848
Andrade-Vieira R, Goguen D, Bentley HA et al (2014) Pre-clinical study of drug combinations that reduce breast cancer burden due to aberrant mTOR and metabolism promoted by LKB1 loss. Oncotarget 5:12738–12752
Vijayaraghavan R, Kumar D, Dube SN et al (2006) Acute toxicity and cardio-respiratory effects of 2-deoxy-D-glucose: a promising radio sensitiser. Biomed Environ Sci 19:96–103
Zhu XX, Ding YH, Wu Y et al (2016) Silibinin: a potential old drug for cancer therapy. Expert Rev Clin Pharmacol 9(10):1323–1330
Pirouzpanah MB, Sabzichi M, Pirouzpanah S et al (2015) Silibilin-induces apoptosis in breast cancer cells by modulating p53, p21, Bak and Bcl-XL pathways. Asian Pac J Cancer Prev 16:2087–2092
Flaig TW, Gustafson DL, Su LJ et al (2007) A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs 25:139–146
Jia L, Huang S, Yin X et al (2018) Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci 208:123–130
Kasiri N, Rahmati M, Ahmadi L et al (2020) Therapeutic potential of quercetin on human breast cancer in different dimensions. Inflammopharmacology 28:39–62
Siebeneicher H, Cleve A, Rehwinkel H et al (2016) Identification and optimization of the First highly selective GLUT1 inhibitor BAY-876. ChemMedChem 11:2261–2271
Wu Q, Ba-Alawi W, Deblois G et al (2020) GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat Commun 11:4205
Chen M, Gowd V, Wang M et al (2021) The apple dihydrochalcone phloretin suppresses growth and improves chemosensitivity of breast cancer cells via inhibition of cytoprotective autophagy. Food Funct 12:177–190
Azevedo C, Correia-Branco A, Araújo JR, Guimarães JT, Keating E, Martel F et al (2015) The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr Cancer 67: 504–513
Toschi E, Sgadari C, Malavasi L et al (2011) Human immunodeficiency virus protease inhibitors reduce the growth of human tumors via a proteasome-independent block of angiogenesis and matrix metalloproteinases. Int J Cancer 128:82–93
Fumarola C, Caffarra C, la Monica S et al (2013) Effects of Sorafenib on energy metabolism in breast cancer cells: role of AMPK-mTORC1 signaling. Breast Cancer Res Treat 141:67–78
**ntaropoulou C, Ward C, Wise A et al (2015) A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 6:25677–25695
Qian Y, Wang X, Liu Y et al (2014) Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett 351:242–251
De A, Wadhwani A, Sauraj et al (2023) WZB117 decorated metformin-carboxymethyl Chitosan nanoparticles for targeting breast Cancer metabolism. Polym (Basel) 15:976
Chen Q, Meng YQ, Xu XF, Gu J (2017) Blockade of GLUT1 by WZB117 resensitizes breast cancer cells to adriamycin. Anticancer Drugs 28:880–887
Dwarakanath B, Jain V (2009) Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol 5:581–585
Di Cosimo S, Ferretti G, Papaldo P et al (2003) Lonidamine: efficacy and safety in clinical trials for the treatment of solid tumors. Drugs Today 39:157
Price GS, Page RL, Riviere JE et al (1996) Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemother Pharmacol 38:129–135
Liu Z, Zhang YY, Zhang QW et al (2014) 3-Bromopyruvate induces apoptosis in breast cancer cells by downregulating Mcl-1 through the PI3K/Akt signaling pathway. Anticancer Drugs 25:447–455
Tao L, Wei L, Liu Y et al (2017) Gen-27, a newly synthesized flavonoid, inhibits glycolysis and induces cell apoptosis via suppression of hexokinase II in human breast cancer cells. Biochem Pharmacol 125:12–25
Masoud GN, Li W (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389
Wang ZH, Peng WB, Zhang P et al (2021) Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine 73:103627
Gadelha ICN, Fonseca NBS, Oloris SCS et al (2014) Gossypol toxicity from cottonseed products. Sci World J 2014: 231635
Kenar JA (2006) Reaction chemistry of gossypol and its derivatives. J Am Oil Chem Soc 83:269–302
Gao P, Bauvy C, Souquère S et al (2010) The Bcl-2 homology domain 3 mimetic gossypol induces both Beclin 1-dependent and Beclin 1-independent cytoprotective autophagy in cancer cells. The Journal of biological chemistry. 2010, 285, 25570–25581
Zhou M, Zhao Y, Ding Y et al (2010) Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer 9:33
Hong CS, Graham NA, Gu W et al (2016) MCT1 modulates cancer cell pyruvate export and growth of tumors that co-express MCT1 and MCT4. Cell Rep 14:1590–1601
Pinheiro C, Albergaria A, Paredes J et al (2010) Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology 56:860–867
Benyahia Z, Blackman MCNM, Hamelin L et al (2021) In Vitro and in vivo characterization of MCT1 inhibitor AZD3965 confirms Preclinical Safety compatible with breast Cancer Treatment. Cancers 13:569
Chang X, Obianwuna UE, Wang J et al (2023) Glycosylated proteins with abnormal glycosylation changes are potential biomarkers for early diagnosis of breast cancer. Int J Biol Macromol 236:123855
Neelamegham S, Mahal LK (2016) Multi-level regulation of cellular glycosylation: from genes to transcript to enzyme to structure. Curr Opin Struct Biol 40:145–152
Moremen KW, Tiemeyer M, Nairn AV (2012) Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol 13:448–462
Helenius A, Markus A (2001) Intracellular functions of N-linked glycans. Science 291:2364–2369
Cornelissen LA, Van Vliet SJ (2016) A bitter sweet symphony: immune responses to altered O-glycan epitopes in cancer. Biomolecules 6:26
Nardy AFFR, Freire-de-Lima L, Freire-de-Lima GG, Morrot A (2016) The sweet side of immune evasion: role of glycans in the mechanisms of cancer progression. Front Oncol 6:54
Li X, Wang X, Tan Z et al (2016) Role of glycans in cancer cells undergoing epithelial-mesenchymal transition. Front Oncol 6:33
Pinho SS, Reis CA (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 15:540–555
Varki A (2017) Biological roles of glycans. Glycobiology 27:3–49
Tan FY, Tang CM, Exley RM (2015) Sugar coating: bacterial protein glycosylation and host-microbe interactions. Trends Biochem Sci 40:342–350
Corfield AP, Berry M (2015) Glycan variation and evolution in the eukaryotes. Trends Biochem Sci 40:351–359
Duarte HO, Freitas D, Gomes C et al (2016) Mucin-type O-Glycosylation in gastric carcinogenesis. Biomolecules 6:33–42
Spiro RG (2002) Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds, vol 12. Glycobiology, pp 43R–56R
Lee HS, Qi Y, Im W (2015) Effects of N-glycosylation on protein conformation and dynamics: protein data Bank analysis and molecular dynamics simulation study. Sci Rep 5:8926
Solá RJ, Griebenow K (2010) Glycosylation of therapeutic proteins. BioDrugs 24:9–21
Murakami M, Kiuchi T, Nishihara M et al (2016) Chemical synthesis of erythropoietin glycoforms for insights into the relationship between glycosylation pattern and bioactivity. Sci Adv 2:e1500678
Minh Hien N, Izumi M, Sato H et al (2017) Chemical synthesis of glycoproteins with the specific installation of gradient-enriched 15 N-labeled amino acids for getting insights into glycoprotein behavior. Chem Eur J 23:6579–6585
Ressler VT, Raines RT (2019) Consequences of the endogenous N-glycosylation of human ribonuclease 1. Biochemistry 58:987–996
Itano N, Iwamoto S (2023) Dysregulation of hexosamine biosynthetic pathway wiring metabolic signaling circuits in cancer. Biochim Biophys Acta Gen Subj 1867:130250
Liu Y, Yu K, Zhang K et al (2023) O-GlcNAcylation promotes topoisomerase IIα catalytic activity in breast cancer chemoresistance. EMBO Rep 24(7), e56458
Ma Z, Vosseller K (2014) Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J Biol Chem 289:34457–34465
Liu Y, Yu K, Kong X et al (2023) FOXA1 O-GlcNAcylation-mediated transcriptional switch governs metastasis capacity in breast cancer. Sci Adv 9(33):eadg7112
Barkovskaya A, Seip K, Hilmarsdottir B et al (2019) O-GlcNAc transferase inhibition differentially affects breast Cancer subtypes. Sci Rep 9:5670
Inman JL, Robertson C, Mott JD, Bissell MJ (2015) Mammary gland development: cell fate specification. stem Cells Microenvironment Dev 142:1028–1042
Nath S, Mukherjee P (2014) MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med 20:332–342
Bose M, Mukherjee P (2020) Microbe-MUC1 crosstalk in Cancer-Associated infections. Trends Mol Med 26:324–336
Carson DD (2008) The cytoplasmic tail of MUC1: a very busy place. Sci Signal 1:pe35
Chen W, Zhang Z, Zhang S et al (2021) MUC1: structure, function, and clinic application in epithelial cancers. Int J Mol Sci 22:12
Kim SH, Turnbull J, Guimond S (2011) Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 209:139–151
Schwartz MA, Ginsberg MH (2002) Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 4:E65–E68
Janik ME, Przybylo M, Pochec E et al (2010) Effect of alpha3beta1 and alphavbeta3 integrin glycosylation on interaction of melanoma cells with vitronectin. Acta Biochim Pol 57:55–61
Singh C, Shyanti RK, Singh V et al (2018) Integrin expression and glycosylation patterns regulate cell-matrix adhesion and alter with breast cancer progression. Biochem Biophys Res Commun 499:374–380
Huanna T, Tao Z, **angfei W et al (2015) GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol Carcinog 54:1159–1171
Xu C, Zhang M, Bian L et al (2020) N-glycosylated SGK196 suppresses the metastasis of basal-like breast cancer cells. Oncogenesis 9:4
Costa AF, Campos D, Reis CA, Gomes C (2020) Targeting glycosylation: a New Road for Cancer Drug Discovery. Trends Cancer 6:757–766
Bertucci F, Gonçalves A (2017) Immunotherapy in breast Cancer: the emerging role of PD-1 and PD-L1. Curr Oncol Rep 19:64
Yang YH, Liu JW, Lu C, Wei JF (2022) CAR-T cell therapy for breast Cancer: from Basic Research to Clinical Application. Int J Biol Sci 18:2609–2626
Yang T, Kang L, Li D, Song Y (2023) Immunotherapy for HER-2 positive breast cancer. Front Oncol 13:1097983
Liu H, Ma L, Lin J et al (2020) Advances in molecular mechanisms of drugs affecting abnormal glycosylation and metastasis of breast cancer. Pharmacol Res 155:104738
Landuyt LV, Lonigro C, Meuris L, Callewaert N (2019) Customized protein glycosylation to improve biopharmaceutical function and targeting. Curr Opin Biotechnol 60:17–28
Chen F, Huang G (2019) Application of glycosylation in targeted drug delivery. Eur J Med Chem 182:111612
Ricciardiello F, Bergamaschi L, De Vitto H et al (2021) Suppression of the HBP function increases pancreatic cancer cell sensitivity to a pan-RAS inhibitor. Cells 10:431
Hang HC, Bertozzi CR (2001) Ketone isosteres of 2-N-acetamidosugars as substrates for metabolic cell surface engineering. J Am Chem Soc 123:1242–1243
Rabuka D, Hubbard SC, Laughlin ST et al (2006) A chemical reporter strategy to probe glycoprotein fucosylation. J Am Chem Soc 128:12078–12079
Berthe A, Zafno M, Muller C et al (2018) Protein N-glycosylation alteration and glycolysis inhibition both contribute to the antiproliferative action of 2-deoxyglucose in breast cancer cells. Breast Cancer Res Treat 171:581–591
Ricciardiello F, Votta G, Palorini R et al (2018) Inhibition of the hexosamine biosynthetic pathway by targeting PGM3 causes breast cancer growth arrest and apoptosis. Cell Death Dis 9:1–17
Zada S, Hwang JS, Ahmed M et al (2019) Protein kinase A activation by βLapachone is associated with apoptotic cell death in NQO1overexpressing breast cancer cells. Oncol Rep 42:1621–1630
Peiris D, Spector AF, Lomax-Browne H et al (2017) Cellular glycosylation affects herceptin binding and sensitivity of breast cancer cells to doxorubicin and growth factors. Sci Rep 7:43006
Crozier Jennifer A, LaPlant B, Timothy H et al (2016) A phase II trial of irinotecan with cetuximab in patients with metastatic breast Cancer previously exposed to anthracycline and/or taxane-containing therapy. Clin Breast Cancer 16:23–30
Zhang Y, Yang ND, Zhou F et al (2012) (-)-Epigallocatechin-3-Gallate induces non-apoptotic cell death in human cancer cells via ROS-mediated lysosomal membrane permeabilization. PLoS ONE 7:e46749
Zan L, Chen Q, Zhang L, Li X (2019) Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 10:374–382
Hong OY, Noh EM, Jang HY et al (2017) Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol Lett 4:441–446
Wang L, Li P, Feng K (2023) EGCG adjuvant chemotherapy: current status and future perspectives. Eur J Med Chem 250:115197
Kim BM, Kim DH, Park JH (2013) Ginsenoside Rg3 induces apoptosis of human breast Cancer (MDA-MB-231) cells. J Cancer Prev 18(2):177–185
Agarwal A, Klueh U, Shih SC et al (2004) N-acetyl-cysteine promotes angiostatin production and vascular collapse in an orthotopic model of breast cancer. Am J Pathol 164:1683–1696
Almaraz RT, Tian Y, Bhattarcharya R et al (2012) Metabolic flux increases glycoprotein sialylation: implications for cell adhesion and cancer metastasis. Mol Cell Prot 11:M112
Nagel AK, Ball LE (2015) Intracellular protein O-GlcNAc modification integrates nutrient status with transcriptional and metabolic regulation. Adv Cancer Res 126:137–166
Lee JB, Pyo KH, Kim HR (2021) Role and function of O-GlcNAcylation in cancer. Cancers 13:5365
Yi W, Clark PM, Mason DE et al (2012) Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337:975e980
Paneque A, Fortus H, Zheng J et al (2023) The Hexosamine Biosynthesis Pathway: regulation and function. Genes (Basel) 14:933
de Queiroz RM, Oliveira IA, Piva B et al (2019) Hexosamine Biosynthetic Pathway and Glycosylation Regulate Cell Migration in Melanoma cells. Front Oncol 9:116
Jia C, Li H, Fu D, Lan Y (2020) GFAT1/HBP/O-GlcNAcylation Axis Regulates β-Catenin Activity to Promote Pancreatic Cancer Aggressiveness. BioMed Res Int 2020: 1921609
Chokchaitaweesuk C, Kobayashi T, Izumikawa T, Itano N (2019) Enhanced hexosamine metabolism drives metabolic and signaling networks involving hyaluronan production and O-GlcNAcylation to exacerbate breast cancer. Cell Death Dis 10:803
Chanmee T, Ontong P, Izumikawa T et al (2016) Hyaluronan production regulates metabolic and cancer stem-like properties of breast cancer cells via hexosamine biosynthetic pathway-coupled HIF-1 signaling. J Biol Chem 291:24105–24120
Moloughney JG, Vega-Cotto NM, Liu S et al (2018) mTORC2 modulates the amplitude and duration of GFAT1 Ser-243 phosphorylation to maintain flux through the hexosamine pathway during starvation. J Biol Chem 293:16464–16478
Acknowledgements
We would like to thank the discussion and language editing by Prof. Song Li from Dalian Medical University.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC 32170499 and 32070440).
Author information
Authors and Affiliations
Contributions
ZJ and SH drafted the main manuscript text; ZJ prepared figures; ZJ and WC edited and finalized the manuscript; WC and SD designed and supervised the whole project. All authors reviewed the manuscript and approved the submission.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J., Sun, H., Wang, C. et al. Breast cancer therapy: from the perspective of glucose metabolism and glycosylation. Mol Biol Rep 51, 546 (2024). https://doi.org/10.1007/s11033-024-09466-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09466-w