Abstract
Background
Genome editing technology has become one of the excellent tools for precise plant breeding to develop novel plant germplasm. The Tobacco mosaic virus (TMV) is the most prominent pathogen that infects several Solanaceae plants, such as tobacco, tomato, and capsicum, which requires critical host factors for infection and replication of its genomic RNA in the host. The Tobamovirus multiplication (TOM) genes, such as TOM1, TOM2A, TOM2B, and TOM3, are involved in the multiplication of Tobamoviruses. TOM1 is a transmembrane protein necessary for efficient TMV multiplication in several plant species. The TOM genes are crucial recessive resistance genes that act against the tobamoviruses in various plant species.
Methods and results
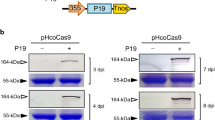
The single guided RNA (sgRNA) was designed to target the first exon of the NtTOM1 gene and cloned into the pHSE401 vector. The pHSE401-NtTOM1 vector was introduced into Agrobacterium tumefaciens strain LBA4404 and then transformed into tobacco plants. The analysis on T0 transgenic plants showed the presence of the hptII and Cas9 transgenes. The sequence analysis of the NtTOM1 from T0 plants showed the indels. Genotypic evaluation of the NtTOM1 mutant lines displayed the stable inheritance of the mutations in the subsequent generations of tobacco plants. The NtTOM1 mutant lines successfully conferred resistance to TMV.
Conclusions
CRISPR/Cas genome editing is a reliable tool for investigating gene function and precision breeding across different plant species, especially the species in the Solanaceae family.







Similar content being viewed by others
Data Availability
Not applicable.
References
Jones RAC (2021) Global plant virus disease pandemics and epidemics. Plants 10:233. https://doi.org/10.3390/plants10020233
Hull R (2014) Mathews’ Plant Virology, 5th ed.; Academic Press: London, UK, 2014
Adams MJ, Adkins S, Bragard C, Gilmer D, Li D, MacFarlane SA, Wong SM, Melcher U, Ratti C, Ryu KH (2017) ICTV Virus taxonomy profile: Virgiviridae. J Gen Vir 98:1999–2000. https://doi.org/10.1099/jgv.0.000884
ICTV Virus Taxonomy (2019) Release. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 9th Dec 2021)
Luria N, Smith E, Reingold V, Bekelman I, Lapidot M, Levin I, Elad N, Tam Y, Sela N, Abu-Ras A, Ezra N (2017) A new israeli Tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE 12(1):e0170429
Dorokhov YL, Sheshukova EV, Komarova TV (2017) Tobamovirus 3′-terminal gene overlap may be a mechanism for within-host fitness improvement. Front Microbiol 8:851. https://doi.org/10.3389/fmicb.2017.00851
Hashimoto M, Neriya Y, Yamaji Y, Namba S (2016) Recessive resistance to plant viruses: potential resistance genes beyond translation initiation factors. Front Microbiol 7:1695
Yamanaka T, Ohta T, Takahashi M, Meshi T, Schmidt R, Dean C, Naito S, Ishikawa M (2000) TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc Natl Acad Sci USA 97:10107–10112
Tsujimoto Y, Numaga T, Ohshima K, Yano MA, Ohsawa R, Goto DB, Naito S, Ishikawa M (2003) Arabidopsis TOBAMOVIRUS MULTIPLICATION (TOM) 2 locus encodes a transmembrane protein that interacts with TOM1. EMBO J 22(2):335–343
Nishikiori M, Mori M, Dohi K, Okamura H, Katoh E, Naito S, Meshi T, Ishikawa M (2011) A host small GTP-binding protein ARL8 plays crucial roles in tobamovirus RNA replication. PLoS Path 7(12):e1002409
Hancinsky R, Mihalik D, Mrkvova M, Candresse T, Glasa M (2020) Plant viruses infecting Solanaceae family members in the cultivated and wild environments: a review. Plants 9:667. https://doi.org/10.3390/plants9050667
Hagiwara Y, Komoda K, Yamanaka T, Tamai A, Meshi T, Funada R, Tsuchiya T, Naito S, Ishikawa M (2003) Subcellular localization of host and viral proteins associated with tobamovirus RNA replication. EMBO J 22(2):344–353
Chen B, Jiang JH, Zhou XP (2007) A TOM1 homologue is required for multiplication of Tobacco mosaic virus in Nicotiana benthamiana. J Zhejiang Univ Sci B 8(4):256–259. https://doi.org/10.1631/jzus.2007.B0256
Zhao Y, Yang X, Zhou G, Zhang T (2020) Engineering plant virus resistance: from RNA silencing to genome editing strategies. Plant Biotech J 18:328–336
Yoon Y-J, Venkatesh J, Lee J-H, Kim J, Lee H-E, Kim D-S, Kang B-C (2020) Genome editing of eIF4E1 in tomato confers resistance to Pepper Mottle Virus. Front Plant Sci 11:1098. https://doi.org/10.3389/fpls.2020.01098
Hagiwara KY, Hirai K, Mochizuki A, Nishiguchi M, Meshi T, Ishikawa M (2008) Overexpression of a host factor TOM1 inhibits tomato mosaic virus propagation and suppression of RNA silencing. Virology 376(1):132–139
Kumar S, Dubey AK, Karmakar R, Kini KR, Mathew MK, Prakash HS (2012) Inhibition of TMV multiplication by siRNA constructs against TOM1 and TOM3 genes of Capsicum annuum. J Virol Meth 186(1–2):78–85
Ahmad S, Wei X, Sheng Z, Hu P, Tang S (2020) CRISPR/Cas9 for development of disease resistance in plants: recent progress, limitations and future prospects. Brief Functl Genom 19(1):26–39. https://doi.org/10.1093/bfgp/elz041
Rato C, Carvalho MF, Azevedo C, Oblessuc PR (2021) Genome editing for resistance against plant pests and pathogens. Trans Res 30:427–459
Pyott DE, Sheehan E, Molnar A (2016) Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol Plant Pathol 17:1276–1288
Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M, Sherman A, Arazi T, Gal-On A (2016) Development of broad virus resistance in non‐transgenic cucumber using CRISPR/Cas9 technology. Mole Plant Path 17(7):1140–1153
Macovei A, Sevilla NR, Cantos C, Jonson GB, Slamet-Loedin I, Cermak T, Voytas DF, Choi IR, Chadha‐Mohanty P (2018) Novel alleles of rice eIF4G generated by CRISPR/Cas9‐targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotech J 16(11):1918–1927. https://doi.org/10.1111/pbi.12927
Gomez MA, Lin ZD, Moll T, Chauhan RD, Hayden L, Renninger K, Beyene G, Taylor NJ, Carrington JC, Staskawicz BJ, Bart RS (2019) Simultaneous CRISPR/Cas9-mediated editing of cassava eIF 4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotech J 17:421–434. https://doi.org/10.1111/pbi.12987
Tripathi JN, Ntui VO, Ron M et al (2019) CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun Biol 2:46. https://doi.org/10.1038/s42003-019-0288-7
Atarashi H, Jayasinghe WH, Kwon J, Kim H, Taninaka Y, Igarashi M, Ito K, Yamada T, Masuta C, Nakahara KS (2020) Artificially edited alleles of the eukaryotic translation initiation factor 4E1 gene differentially reduce susceptibility to Cucumber mosaic virus and Potato virus Y in tomato. Front Microbiol 11:3075
Pramanik D, Shelake RM, Park J, Kim MJ, Hwang I, Park Y, Kim JY (2021) CRISPR/Cas9-mediated generation of pathogen-resistant tomato against tomato yellow leaf curl virus and powdery mildew. Inter J Mol Sci 22(4):1878
Kumar S, Abebie B, Kumari R, Kravchik M, Shnaider Y, Leibman D, Bornstein M, Gaba V, Gal–On A (2022) Development of PVY resistance in tomato by knockout of host eukaryotic initiation factors by CRISPR–Cas9. Phytoparasitica 50:743–756
Lucioli A, Tavazza R, Baima S, Fatyol K, Burgyan J, Tavazza M (2022) CRISPR-Cas9 targeting of the eIF4E1 gene extends the Potato Virus Y resistance spectrum of the Solanum tuberosum L. cv. Desiree Front Microbiol 13:873930. https://doi.org/10.3389/fmicb.2022.873930
Kuroiwa K, Thenault C, Nogue F, Perrot L, Mazier M, Gallois JL (2022) CRISPR-based knock-out of eIF4E2 in a cherry tomato background successfully recapitulates resistance to pepper veinal mottle virus. Plant Sci 316:111160
**ng HL, Dong L. Wang ZP, Zhang HY, Han CY, Liu B, … Chen QJA (2014) CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biology 14(1):1–12
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12(13):39–40
Hooghvorst I, Lopez-Cristoffanini C, Nogues S (2019) Efficient knockout of phytoene desaturase gene using CRISPR/Cas9 in melon. Sci Rep 9(1):1–7
Yamanaka T, Imai T, Satoh R, Kawashima A, Takahashi M, Tomita K, Kubota K, Meshi T, Naito S, Ishikawa M (2002) Complete inhibition of tobamovirus multiplication by simultaneous mutations in two homologous host genes. J Virol 76(5):2491–2497
Ali ME, Ishii Y, Taniguchi JI, Waliullah S, Kobayashi K, Yaeno T, … Nishiguchi M (2018)Conferring virus resistance in tomato by independent RNA silencing of three tomato homologs of Arabidopsis TOM1. Arch. Virology 163(5):1357–1362
Cao Y, Zhou H, Zhou X, Li F (2020) Control of plant viruses by CRISPR/Cas system-mediated adaptive immunity. Front Microbiol 11:593700. https://doi.org/10.3389/fmicb.2020.593700
Gal-On A, Fuchs M, Gray S (2017) Generation of novel resistance genes using mutation and targeted gene editing. Curr Opin Virol 26:98–103
Zhang DQ, Unver T, Zhang BH (2021) CRISPR/Cas: a powerful tool for gene function study and crop improvement. J Adv Res 29:207–221
Li C, Unver T, Zhang BH (2017) A high-efficiency CRISPR/Cas9 system for targeted mutagenesis in cotton (Gossypium hirsutum L). Sci Rep 7:43902
Alok A, Chauhan H, Kaushal N, Singh K (2022) Rapid and efficient CRISPR/Cas9-mediated genome editing in potato via hairy root induction. In Vitro Cellular & Developmental Biology-Plant.1–12
Robertson G, Burger J, Campa M (2022) CRISPR/Cas-based tools for the targeted control of plant viruses. Mol Plant Pathol 23:1701–1718. https://doi.org/10.1111/mpp.13252
Ariga H, Toki S, Ishibashi K (2020) Potato virus X vector-mediated DNA-free genome editing in plants. Plant Cell Physiol 61(11):1946–1953. https://doi.org/10.1093/pcp/pcaa123
van de Vossenberg BTLH, Dawood T, Wozny M, Botermans M (2021) First expansion of the Public Tomato Brown Rugose Fruit Virus (ToBRFV) Nextstrain Build; inclusion of new genomic and epidemiological data. PhytoFrontiers™ 1:359–363
Ishikawa M, Yoshida T, Matsuyama M, Kouzai Y, Kano A, Ishibashi K (2022) Tomato brown rugose fruit virus resistance generated by quadruple knockout of homologs of TOBAMOVIRUS MULTIPLICATION1 in tomato. Plant Physiol 189:679–686
Kravchik M, Shnaider Y, Abebie B, Shtarkman M, Kumari R, Kumar S, Leibman D, Spiegelman Z, Gal-On A (2022) Knockout of SlTOM1 and SlTOM3 results in differential resistance to tobamovirus in tomato. Mol Plant Pathol 23:1278–1289. https://doi.org/10.1111/mpp.13227
Acknowledgements
PJ and VRA are grateful to the University Grant Commission, New Delhi, for the financial assistance under the SAP-DRS-II program (No. F-5-24/2015/DRS-II). DS is thankful to the Department of Science and Technology, Government of India, for providing Inspire Fellowship (No. IF160264). SA is grateful to the Council of Scientific & Industrial Research, Government of India, for giving CSIR-Emeritus Scientist (No. 21(1067)/19/EMR-II).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
PJ performed experiments and wrote the manuscript. DS helped while conducting experiments and data analysis. AA helped in bioinformatics, construct, and manuscript preparation. VP and SPS data curation and revision of the manuscript. SA experimental suggestions and critical revision of the manuscript, BZ and VRA conceived the idea, designed the experiments, and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Institutional Review Board Statement
Not applicable.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
The authors agree to publication in the Journal.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jogam, P., Sandhya, D., Alok, A. et al. Editing of TOM1 gene in tobacco using CRISPR/Cas9 confers resistance to Tobacco mosaic virus. Mol Biol Rep 50, 5165–5176 (2023). https://doi.org/10.1007/s11033-023-08440-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08440-2