Abstract
Background
Peach (Prunus persica L.) is prone to chilling injury as exhibited by inhibition of the ethylene production, failure in softening, and the manifestation of internal browning. The basic leucine zipper (bZIP) transcription factors play an essential role in regulatory networks that control many processes associated with physiological, abiotic and biotic stress responses in fruits. Formerly, the underlying molecular and regulatory mechanism of (bZIP) transcription factors responsive to chilling injury in peach fruit is still elusive.
Methods and results
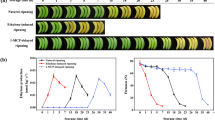
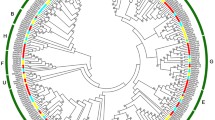
In the current experiment, the solute peach ‘Zhongyou Peach No. 13’ was used as the test material and cold storage at low temperature (4 °C). It was found that long-term low-temperature storage induced the production of ethylene, the hardness of the pulp decreased, and the low temperature also induced ABA accumulation. The changes of ABA and ethylene in peach fruits during low-temperature storage were clarified. Since the bZIP transcription factor is involved in the regulation of downstream pathways of ABA signals, 47 peach bZIP transcription factor family genes were identified through bioinformatics analysis. Further based on RT-qPCR analysis, 18 PpbZIP genes were discovered to be expressed in refrigerated peach fruits. Among them, the expression of PpbZIP23 and PpbZIP25 was significantly reduced during the refrigeration process, the promoter analysis of these genes found that this region contains the MYC/MYB/ABRES binding element, but not the DRES/CBFS element, indicating that the expression may be regulated by the ABA-dependent cold induction pathway, thereby responding to chilling injury in peach fruit.
Conclusions
Over investigation will provide new insights for further postharvest protocols related to molecular changes during cold storage and will prove a better cope for chilling injury.







Similar content being viewed by others
Data availability
All supporting data is available within the text and supplementary files. Further queries can be directed to corresponding author.
References
Wang K, Yin XR, Zhang B, Grierson D, Xu CJ, Chen KS (2017) Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit. Plant Cell Environ 40(8):1531–1551
Wang L, Shan T, **e B, Ling C, Shao S, ** P, Zheng Y (2019) Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem 272:530–538
Lurie S, Crisosto CH (2005) Chilling injury in peach and nectarine. Postharvest Biol Technol 37(3):195–208
Park S, Lee CM, Doherty CJ, Gilmour SJ, Kim Y, Thomashow MF (2015) Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J 82(2):193–207
Zhu J, Dong CH, Zhu JK (2007) Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol 10(3):290–295
Gao SQ, Chen M, Xu ZS, Zhao CP, Li L, Xu HJ, Tang YM, Zhao X, Ma YZ (2011) The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol Biol 75(6):537–553
Kim JB, Kang JY, Kim SY (2004) Over-expression of a transcription factor regulating ABA-responsive gene expression confers multiple stress tolerance. Plant Biotechnol J 2(5):459–466
Liao Y, Zou HF, Wei W, Hao YJ, Tian AG, Huang J, Liu YF, Zhang JS, Chen SY (2008) Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 228(2):225–240
Zhang Y, Raza A, Huang H, Su W, Luo D, Zeng L, Ding X, Cheng Y, Liu Z, Li Q (2022) Analysis of Lhcb gene family in rapeseed (Brassica napus L.) identifies a novel member “BnLhcb3. 4” modulating cold tolerance. Environ Exp Bot 198:104848
Raza A, Tabassum J, Kudapa H, Varshney RK (2021) Can omics deliver temperature resilient ready-to-grow crops? Crit Rev Biotechnol 41(8):1209–1232
Wang Z, Wang Y, Li A, Miao Y, Deng L, Wang H, Meng J, Liu H, Niu L, Pan L (2022) Transcriptome, lipidome, and phytohormone analyses elucidate the mechanism of chilling injury during postharvest cold storage in different cold-sensitive peach fruit. Available at SSRN 4085272
Sharif R, Raza A, Chen P, Li Y, El-Ballat EM, Rauf A, Hano C, El-Esawi MA (2021) HD-ZIP gene family: potential roles in improving plant growth and regulating stress-responsive mechanisms in plants. Genes 12(8):1256
Zahra N, Shaukat K, Hafeez MB, Raza A, Hussain S, Chaudhary MT, Akram MZ, Kakavand SN, Saddiq MS, Wahid A (2021) Physiological and molecular responses to high, chilling, and freezing temperature in plant growth and production consequences and mitigation possibilities. Harsh Environment and Plant Resilience. Springer, Cham, pp 235–290
Pérez-Rodríguez P, Riano-Pachon DM, Corrêa LGG, Rensing SA, Kersten B, Mueller-Roeber B (2010) PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res 38(suppl_1):D822–D827
Talanian RV, McKnight CJ, Kim PS (1990) Sequence-specific DNA binding by a short peptide dimer. Science 249(4970):769–771
Hurst HC (1995) Transcription factors 1: bZIP proteins. Protein Profile 2(2):101–168
Jakoby M, Weisshaar B, Dröge-Laser W, Vicente-Carbajosa J, Tiedemann J, Kroj T, Parcy F (2002) bZIP transcription factors in Arabidopsis. Trends Plant Sci 7(3):106–111
Nijhawan A, Jain M, Tyagi AK, Khurana JP (2008) Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol 146(2):333–350
Wang J, Zhou J, Zhang B, Vanitha J, Ramachandran S, Jiang SY (2011) Genome-wide expansion and expression divergence of the basic leucine zipper transcription factors in higher plants with an emphasis on Sorghum F. J Integr Plant Biol 53(3):212–231
Wei K, Chen J, Wang Y, Chen Y, Chen S, Lin Y, Pan S, Zhong X, **e D (2012) Genome-wide analysis of bZIP-encoding genes in maize. DNA Res 19(6):463–476
** Z, Xu W, Liu A (2014) Genomic surveys and expression analysis of bZIP gene family in castor bean (Ricinus communis L.). Planta 239(2):299–312
Baloglu MC, Eldem V, Hajyzadeh M, Unver T (2014) Genome-wide analysis of the bZIP transcription factors in cucumber. PLoS ONE 9(4):e96014
Gao M, Zhang H, Guo C, Cheng C, Guo R, Mao L, Fei Z, Wang X (2014) Evolutionary and expression analyses of basic zipper transcription factors in the highly homozygous model grape PN40024 (Vitis vinifera L.). Plant Mol Biol Report 32(5):1085–1102
Wang XL, Chen X, Yang TB, Cheng Q, Cheng ZM (2017) Genome-wide identification of bZIP family genes involved in drought and heat stresses in strawberry (Fragaria vesca L). Int J Genom. https://doi.org/10.1155/2017/3981031
Zhao J, Guo R, Guo C, Hou H, Wang X, Gao H (2016) Evolutionary and expression analyses of the apple basic leucine zipper transcription factor family. Front Plant Sci 7:376
Weltmeier F, Ehlert A, Mayer CS, Dietrich K, Wang X, Schütze K, Alonso R, Harter K, Vicente-Carbajosa J, Dröge-Laser W (2006) Combinatorial control of Arabidopsis proline dehydrogenase transcription by specific heterodimerisation of bZIP transcription factors. EMBO J 25(13):3133–3143
**ang Y, Tang N, Du H, Ye H, **ong L (2008) Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148(4):1938–1952
Ying S, Zhang D-F, Fu J, Shi Y-S, Song Y-C, Wang T-Y, Li Y (2012) Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis. Planta 235(2):253–266
Liu C, Wu Y, Wang X (2012) bZIP transcription factor OsbZIP52/RISBZ5: a potential negative regulator of cold and drought stress response in rice. Planta 235(6):1157–1169
Shimizu H, Sato K, Berberich T, Miyazaki A, Ozaki R, Imai R, Kusano T (2005) LIP19, a basic region leucine zipper protein, is a Fos-like molecular switch in the cold signaling of rice plants. Plant Cell Physiol 46(10):1623–1634
Nieva C, Busk PK, Domínguez-Puigjaner E, Lumbreras V, Testillano PS, Risueño MC, Pagès M (2005) Isolation and functional characterisation of two new bZIP maize regulators of the ABA responsive gene rab28. Plant Mol Biol 58(6):899–914
Thurow C, Schiermeyer A, Krawczyk S, Butterbrodt T, Nickolov K, Gatz C (2005) Tobacco bZIP transcription factor TGA2. 2 and related factor TGA2. 1 have distinct roles in plant defense responses and plant development. Plant J 44(1):100–113
Ulm R, Baumann A, Oravecz A, Máté Z, Ádám É, Oakeley EJ, Schäfer E, Nagy F (2004) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci U S A 101(5):1397–1402
Silveira AB, Gauer L, Tomaz JP, Cardoso PR, Carmello-Guerreiro S, Vincentz M (2007) The Arabidopsis AtbZIP9 protein fused to the VP16 transcriptional activation domain alters leaf and vascular development. Plant Sci 172(6):1148–1156
Yin Y, Zhu Q, Dai S, Lamb C, Beachy RN (1997) RF2a, a bZIP transcriptional activator of the phloem-specific rice tungro bacilliform virus promoter, functions in vascular development. EMBO J 16(17):5247–5259
Guan Y, Ren H, **e H, Ma Z, Chen F (2009) Identification and characterization of bZIP-type transcription factors involved in carrot (Daucus carota L.) somatic embryogenesis. Plant J 60(2):207–217
Zou M, Guan Y, Ren H, Zhang F, Chen F (2008) A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol Biol 66(6):675–683
Zeng W, Tan B, Deng L, Wang Y, Aslam MM, Wang X, Qiao C, Wang Z, Feng J (2020) Identification and expression analysis of abscisic acid signal transduction genes during peach fruit ripening. Sci Hortic 270:109402
Wang X, Zeng W, Ding Y, Wang Y, Niu L, Yao J-L, Pan L, Lu Z, Cui G, Li G (2019) PpERF3 positively regulates ABA biosynthesis by activating PpNCED2/3 transcription during fruit ripening in peach. Hortic Res. https://doi.org/10.1038/s41438-018-0094-2
Aslam MM, Deng L, Wang X, Wang Y, Pan L, Liu H, Niu L, Lu Z, Cui G, Zeng W (2019) Expression patterns of genes involved in sugar metabolism and accumulation during peach fruit development and ripening. Sci Hortic 257:108633
Zhang C, Shangguan L, Ma R, Sun X, Tao R, Guo L, Korir N, Yu M (2012) Genome-wide analysis of the AP2/ERF superfamily in peach (Prunus persica L). Genet Mol Res 11(4):4789–4809
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Tatsuki M, Nakajima N, Fujii H, Shimada T, Nakano M, Hayashi KI, Hayama H, Yoshioka H, Nakamura Y (2013) Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch). J Exp Bot 64(4):1049–1059
McGlasson W, Scott KJ, Mendoza D Jr (1979) The refrigerated storage of tropical and subtropical products. Int J Refrig 2(6):199–206
Hardeburg RE (1986) The commercial storage of fruits, vegetables, and florist and nursery stocks. USDA Agriculture Handbook, vol 66, pp 11–12
Lallu N (1995) Low temperature breakdown in kiwifruit. In: III International Symposium on Kiwifruit (444): pp 579–586
Mworia EG, Yoshikawa T, Salikon N, Oda C, Fukuda T, Suezawa K, Asiche WO, Ushijima K, Nakano R, Kubo Y (2011) Effect of MA storage and 1-MCP on storability and quality of ‘Sanuki Gold’ kiwifruit harvested at two different maturity stages. J Jpn Soc Hortic Sci 80(3):372–377
Koukounaras A, Sfakiotakis E (2007) Effect of 1-MCP prestorage treatment on ethylene and CO2 production and quality of ‘Hayward’kiwifruit during shelf-life after short, medium and long term cold storage. Postharvest Biol Technol 46(2):174–180
** P, Wang K, Shang H, Tong J, Zheng Y (2009) Low-temperature conditioning combined with methyl jasmonate treatment reduces chilling injury of peach fruit. J Sci Food Agric 89(10):1690–1696
Ritenour MA, Crisosto CH, Garner DT, Cheng GW, Zoffoli JP (1999) Temperature, length of cold storage and maturity influence the ripening rate of ethylene-preconditioned kiwifruit. Postharvest Biol Technol 15(2):107–115
Tijero V, Teribia N, Muñoz P, Munné-Bosch S (2016) Implication of abscisic acid on ripening and quality in sweet cherries: differential effects during pre-and post-harvest. Front Plant Sci 7:602
Li P, Dai S, Zhao B, Zhang Y, Liao K, Xu F, Du C, Leng P (2014) Effect of low temperatures on pulp browning and endogenous abscisic acid and ethylene concentrations in peach (Prunus persica L.) fruit during post-harvest storage. J Hortic Sci Biotechnol 89(6):686–692
Yang Q, Zhang Z, Rao J, Wang Y, Sun Z, Ma Q, Dong X (2013) Low-temperature conditioning induces chilling tolerance in ‘Hayward’ kiwifruit by enhancing antioxidant enzyme activity and regulating en-dogenous hormones levels. J Sci Food Agric 93(15):3691–3699
Alves MS, Dadalto SP, Gonçalves AB, De Souza GB, Barros VA, Fietto LG (2013) Plant bZIP transcription factors responsive to pathogens: a review. Int J Mol Sci 14(4):7815–7828
Sornaraj P, Luang S, Lopato S, Hrmova M (2016) Basic leucine zipper (bZIP) transcription factors involved in abiotic stresses: a molecular model of a wheat bZIP factor and implications of its structure in function. Biochim Biophys Acta (BBA)-General Subj 1860(1):46–56
Liu X, Chu Z (2015) Genome-wide evolutionary characterization and analysis of bZIP transcription factors and their expression profiles in response to multiple abiotic stresses in Brachypodium distachyon. BMC Genom 16(1):227
Liu J, Chen N, Chen F, Cai B, Dal Santo S, Tornielli GB, Pezzotti M, Cheng ZMM (2014) Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera L). BMC Genom 15(1):281
Hu W, Zuo J, Hou X, Yan Y, Wei Y, Liu J, Li M, Xu B, ** Z (2015) The auxin response factor gene family in banana: genome-wide identification and expression analyses during development, ripening, and abiotic stress. Front Plant Sci 6:742
Sevillano L, Sanchez-Ballesta MT, Romojaro F, Flores FB (2009) Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J Sci Food Agric 89(4):555–573
Brummell DA, Dal Cin V, Crisosto CH, Labavitch JM (2004) Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Bot 55(405):2029–2039
Hsieh TH, Li CW, Ruey-Chih S, Cheng CP (2010) A tomato bZIP transcription factor, SlAREB, is involved in water deficit and salt stress response. Planta 231(6):1459–1473
Kesarwani M, Yoo J, Dong X (2007) Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiol 144(1):336–346
Liu C, Ou S, Mao B, Tang J, Wang W, Wang H, Cao S, Schläppi MR, Zhao B, **ao G (2018) Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat Commun 9(1):1–12
Zhang T, Zhao X, Wang W, Pan Y, Huang L, Liu X, Zong Y, Zhu L, Yang D, Fu B (2012) Comparative transcriptome profiling of chilling stress responsiveness in two contrasting rice genotypes. PLoS ONE 7(8):e43274
Yun K-Y, Park MR, Mohanty B, Herath V, Xu F, Mauleon R, Wijaya E, Bajic VB, Bruskiewich R, delosReyes BG (2010) Transcriptional regulatory network triggered by oxidative signals configures the early response mechanisms of japonica rice to chilling stress. BMC Plant Biol 10(1):16
Chen D, Xu G, Tang W, **g Y, Ji Q, Fei Z, Lin R (2013) Antagonistic basic helix-loop-helix/bZIP transcription factors form transcriptional modules that integrate light and reactive oxygen species signaling in Arabidopsis. Plant Cell 25(5):1657–1673
Wang F, Wu N, Zhang L, Ahammed GJ, Chen X, **ang X, Zhou J, **a X, Shi K, Yu J (2018) Light signaling-dependent regulation of photoinhibition and photoprotection in tomato. Plant Physiol 176(2):1311–1326
An JP, Yao JF, Wang XN, You CX, Wang XF, Hao YJ (2017) MdHY5 positively regulates cold tolerance via CBF-dependent and CBF-independent pathways in apple. J Plant Physiol 218:275–281
Soltész A, Smedley M, Vashegyi I, Galiba G, Harwood W, Vágújfalvi A (2013) Transgenic barley lines prove the involvement of TaCBF14 and TaCBF15 in the cold acclimation process and in frost tolerance. J Exp Bot 64(7):1849–1862
Wisniewski M, Norelli J, Artlip T (2015) Overexpression of a peach CBF gene in apple: a model for understanding the integration of growth, dormancy, and cold hardiness in woody plants. Front Plant Sci 6:85
Nakashima K, Ito Y, Yamaguchi-Shinozaki K (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149(1):88–95
**ong L, Ishitani M, Zhu J-K (1999) Interaction of osmotic stress, temperature, and abscisic acid in the regulation of gene expression in Arabidopsis. Plant Physiol 119(1):205–212
Lu G, Gao C, Zheng X, Han B (2009) Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 229(3):605–615
Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17(12):3470–3488
Casaretto J, Ho TH (2003) The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell 15(1):271–284
Kobayashi F, Maeta E, Terashima A, Takumi S (2008) Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings. Physiol Plant 134(1):74–86
**ao-Li S, Li Y, Hua C, ** B, Wei J, Zuo-Jun J, Yan-Ming Z (2011) Arabidopsis bZIP1 transcription factor binding to ABRE cis-element regulates abscisic acid signal transduction. Acta Agron Sin 37(4):612–619
Choi H-i, Hong J-h, Ha J-o, Kang J-y, Kim SY (2000) A family of ABA-responsive element binding factors. J Biol Chem 275(3):1723–1730
Yao L, Hao X, Cao H, Ding C, Yang Y, Wang L, Wang X (2020) ABA-dependent bZIP transcription factor, CsbZIP18, from Camellia sinensis negatively regulates freezing tolerance in Arabidopsis. Plant Cell Rep 39(4):553–565
Kim JC, Lee SH, Cheong YH, Yoo CM, Lee SI, Chun HJ, Yun DJ, Hong JC, Lee SY, Lim CO (2001) A novel cold-inducible zinc finger protein from soybean, SCOF-1, enhances cold tolerance in transgenic plants. Plant J 25(3):247–259
Acknowledgements
This work was supported by the National Natural Science Foundation of China [No. 31872085], the National Key Research and Development Program [2018YFD1000200], and the Agricultural Science and Technology Innovation Program (ASTIP) [CAAS-ASTIP-2019-ZFRI]. Authors are highly grateful for providing financial assistance for this research.
Funding
This work was supported by the National Natural Science Foundation of China [No. 31872085], the National Key Research and Development Program [2018YFD1000200], and the Agricultural Science and Technology Innovation Program (ASTIP) [CAAS-ASTIP-2019-ZFRI].
Author information
Authors and Affiliations
Contributions
WZ conceived this project and designed all the experiment. MMA, LD, YW performed the experiments. JM analyzed data. ZW supervised the experiments MMA and WZ wrote the article, LP, LN, ZL and GC help to revise the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that none of the work contained in this manuscript is published in any language or current under consideration at any other journal; there is no conflict of interest to declare. All authors have contributed to, read, and approved this submitted manuscript in its current form.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslam, M.M., Deng, L., Meng, J. et al. Characterization and expression analysis of basic leucine zipper (bZIP) transcription factors responsive to chilling injury in peach fruit. Mol Biol Rep 50, 361–376 (2023). https://doi.org/10.1007/s11033-022-08035-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08035-3