Abstract
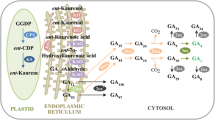
The GH3 genes play vital roles in auxin homeostasis by conjugating excess auxin to amino acids. However, how GH3 genes function during grafting in Chinese hickory (Carya cathayensis) is largely unknown. Here, based on the transcriptome database, a comprehensive identification and expression profiling analysis of 12 GH3 genes in Chinese hickory were performed. Phylogenetic analysis indicated that CcGH3-x exists in a specific subfamily. To understand the roles of CcGH3 genes, tissue-specific expression and the response to different phytohormones were determined. Expression profiles of GH3 genes of Chinese hickory during grafting were analysed. The data suggested that 10 CcGH3 genes were down-regulated at an early stage of grafting, indicating that auxin homeostasis regulated by the CcGH3 family might be inhibited at initial stages. At the completion of grafting, expression levels of members of the CcGH3 family were restored to normal levels. Endogenous auxin levels were also measured, and the data showed that free auxin decreased to the lowest level at an early stage of grafting, and then increased during grafting. Auxin amino acid conjugation increased at an early stage of grafting in rootstock, and then decreased with progression of the graft union. Our results demonstrate that the reduced expression of CcGH3 family genes during grafting might contribute to the release of free auxin, making an important contribution to the recovery of auxin levels after grafting.







Similar content being viewed by others
References
Cheng XJ, Huang JQ, Zheng BS, Ye ZX (2002) Advances in study of Carya cathayensis. J Zhejiang For Sci Technol 03:19–23
Huang JQ, Zheng BS, Huang YJ, **a GH, Zeng YR (2006) Selection and breeding of new varieties of the oil-bearing tree hickory (Caray cathayensis Sarg.). Biomass Chem Eng 1:178–181
Chang J, Ren XH, Wang KL, Teng JH, Zhou JG, Zhou Y, Fu GL (2018) Analysis of fatty acids and amino acid composition in Carya cathayensis clones. J Central South Univ For Technol 38:20–26
**a YJ, **a XH, Ren HD, Wang KL, Chang J, Fu SL, Teng JH, Shao WZ (2018) Comparative analysis of the nutritional components of 22 Carya cathayensis Clones. J Chin Cereals Oils Assoc 33(4):49–55
Lewandowski M, Zurawicz E (2000) Shortening the juvenile period in apple seedings by grafting on P 22 dwarfing rootstock. J Fruit Ornam Plant Res 8(1):33–37
Zheng BS, Liu L, Huang JQ, Cheng XJ, Zhu YQ, Xu HQ (2002) Analysis on physiological and biochemical traits of survival of Carya cathayensis grafted seedling. J Fujian Coll For 22:320–324
Flaishman MA, Loginovsky K, Golobowich S, Lev-Yadun S (2008) Arabidopsis thaliana as a model system for graft union development in homografts and heterografts. J Plant Growth Regul 27:231
Wang Y, Kollmann R (1996) Vascular differentiation in the graft union of in-vitro grafts with different compatibility. -Structural and functional aspects. J Plant Physiol 147(5):521–533
Yin H, Yan B, Sun J, Jia PF, Zhang ZJ, Yan XS, Chai J, Ren ZZ, Zheng GC, Liu H (2012) Graft-union development: a delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. J Exp Bot 63(11):4219–4232
Su W (2016) Research on anatomical, physiological and biochemical traits of union of Carya illinoensis bud grafting. Nan**g Forestry University, Nan**g
Qiu LL, Jiang B, Fang J, Shen YK, Fang ZX, Saravana KRM, Yi KK, Shen CJ, Yan DL, Zheng BS (2016) Analysis of transcriptome in hickory (Carya cathayensis), and uncover the dynamics in the hormonal signaling pathway during graft process. BMC Genom 17(1):935
Sharma A, Zheng BS (2019) Molecular responses during plant grafting and its regulation by auxins, cytokinins, and gibberellins. Biomolecules 9(9):397
Kumar RMS, Gao LX, Yuan HW, Xu DB, Zhao L, Tao SC, Guo WB, Yan DL, Zheng BS, Edqvist J (2018) Auxin enhances grafting success in Carya cathayensis (Chinese hickory). Planta 247:761–772
Li W, Fang C, Krishnan S, Chen JM, Yu H, Angus SM, Merewitz E, Lorenzo KG, Richard JM, Deng ZN, Janice Z, Li Y (2017) Elevated auxin and reduced cytokinin contents in rootstocks improve their performance and grafting success. Plant Biotechnol J 15(12):1556–1565
Matsuoka K, Sugawara E, Aoki R, Takuma K, Teraomorita M, Satoh S, Asahina M (2016) Differential cellular control by cotyledon-derived phytohormones involved in graft reunion of Arabidopsis hypocotyls. Plant Cell Physiol 57(12):2620–2631
Ostrowski M, Jakubowska A (2013) GH3 expression and IAA-amide synthetase activity in pea (Pisum sativum L.) seedlings are regulated by light, plant hormones and auxinic herbicides. J Plant Physiol 170:361–368
Jutta LM (2011) Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot 62(6):1757–1773
Swarup R, Péret B (2012) AUX/LAX family of auxin influx carriers-an overview. Front Plant Sci 3:225
Park JE, Park JY, Kim YS, Staswick PE, Jeon J, Yun Ju, Kim SY, Kim J, Lee YH, Park CM (2007) GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem 282(13):10036–10046
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17(2):616–627
Hagen G, Kleinschmidt A, Guilfoyle T (1984) Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 162:147–153
Jain M, Kaur N, Tyagi AK, Khurana JP (2006) The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct Integr Genom 6(1):36
Feng SG, Yue RQ, Sun T, Yang YJ, Zhang L, Xu MF, Wang HZ, Shen CJ (2015) Genome-wide identification, expression analysis of auxin-responsive GH3 family genes in maize (Zea mays L.) under abiotic stresses. J Integr Plant Biol 57(9):783–795
Yuan HZ, Zhao K, Lei HJ, Shen XJ, Liu Y, Liao X, Li TH (2013) Genome-wide analysis of the GH3 family in apple (Malus × domestica). BMC genomics 14(1):297
Staswick PE, Iskender T, Rowe ML (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14:1405–1415
Staswick PE, Iskender T (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16:2117–2127
Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T, Hasunuma K, Matsui M (2001) DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J 25(2):213–221
Ding XH, Cao YL, Huang LL, Zhao J, Xu CG, Li XH, Wang SP (2008) Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 20(1):228–240
Zhang ZQ, Li Q, Li ZM, Staswick PE, Wang MY, Zhu Y, He ZH (2007) Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol 145(2):450–464
Du H, Wu N, Fu J, Wang SP, Li XH, **ao JH, **ong LZ (2012) A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J Exp Bot 63(18):6467–6480
Teichmann T, Boluarianto WH, Olbrich A, Langenfeldheyser R, Göbel C, Grzeganek P, Feussner I, Hänsch R, Polle A (2008) GH3:GUS reflects cell-specific developmental patterns and stress-induced changes in wood anatomy in the poplar stem. Tree Physiol 28(9):1305–1315
Zhang SW, Li CH, Cao J, Zhang YC, Zhang SQ, **a YF, Sun DY, Sun Y (2009) Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 Activation1. Plant Physiol 151(4):1889–1901
Zhang SN, Wang SK, Xu YX, Yu CL, Shen CJ, Qian Q, Markus G, Jiang DA, Qi YH (2014) The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3–5 and OsBRI1. Plant Cell Environ 38(4):638–654
Cano A, Sánchez-García AB, Albacete A, González-Bayón R, Justamante MS, Ibáñez S, Acosta M, Pérez-Pérez JM (2018) Enhanced conjugation of auxin by GH3 enzymes leads to poor adventitious rooting in carnation stem cuttings. Front Plant Sci 9:566
Liu ZB, Ulmasov T, Shi X, Hagen G, Guilfoyle TJ (1994) Soybean GH3 promoter contains multiple auxin-inducible elements. Plant Cell 6:645–657
Liu KD, Kang BC, Jiang H, Shanna LM, Li HX, Christopher BW, Tim LS, Molly MJ (2005) A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol Biol 58(4):447–464l
Yang YJ, Yue RQ, Sun T, Zhang L, Chen W, Zeng HQ, Wang HZ, Shen CJ (2015) Genome-wide identification, expression analysis of GH3 family genes in Medicago truncatula under stress-related hormones and Sinorhizobium meliloti infection. Appl Microbiol Biotechnol 99(2):841–854
Ostrowski M, Ciarkowska A, Jakubowska A (2016) The auxin conjugate indole-3-acetyl-aspartate affects responses to cadmium and salt stress in Pisum sativum L. J Plant Physiol 191:63–72
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Porebski S, Bailey LG, Baum BR (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15(1):8–15
Zhang XB, Feng BH, Wang HM, Xu X, Shi YF, He Y, Chen Z, Atul PS, Shi L, Wu JL (2018) A substitution mutation in OsPELOTA confers bacterial blight resistance by activating the salicylic acid pathway. J Integr Plant Biol 60(2):160–172
You CC, Zhu HL, Xu BB, Huang WX, Wang SH, Ding YF, Liu ZH, Li GH, Lin C, Ding CQ, Tang S (2016) Effect of removing superior spikelets on grain filling of inferior spikelets in rice. Front Plant Sci 7:1161
Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3–1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61(13):3615–3625
Liu CH, Hong J, **a GH, Huang JQ (2009) Cytological observation on healing responses in grafting of Carya cathayensis. Sci silvae Sin 45(6):34–38
Zheng BS, Chu HL, ** SH, Huang YJ, Wang ZJ, Chen M, Huang JQ (2010) cDNA-AFLP analysis of gene expression in hickory (Carya cathayensis) during graft process. Tree Physiol 30(2):297–303
Xu DB, Yuan HW, Tong YF, Zhao L, Qiu LL, Guo WB, Shen CJ, Liu HJ, Yan DL, Zheng BS (2017) Comparative proteomic analysis of the graft unions in hickory (Carya cathayensis) provides insights into response mechanisms to grafting process. Front Plant Sci 8:676
Köse K, Güleryüz M (2006) Effects of auxins and cytokinins on graft union of grapevine (Vitis vinifera). N Z J Crop Hortic Sci 34(2):145–150
Kumar R, Agarwal P, Tyagi AK, Sharma AK (2012) Genome-wide investigation and expression analysis suggest diverse roles of auxin-responsive GH3 genes during development and response to different stimuli in tomato (Solanum lycopersicum). Mol Genet Genomics 287(3):221–235
Paponov IA, Paponov M, Teale W, Menges M, Chakrabortee S, Murray JAH, Palme K (2008) Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol Plant 1(2):321–337
Huang JQ, Zhang BS, Lu JW, Fu GW (2001) Anatomical observation on grafting healing progress of Carya cathayensis. J Zhejiang For Coll 18:111–114
Aloni R (2010) The induction of vascular tissues by auxin. Plant hormones. Springer, Dordrecht
Yoshida S, Iwamoto K, Demura T, Fukuda H (2009) Comprehensive analysis of the regulatory roles of auxin in early transdifferentiation into xylem cells. Plant Mol Biol 70(4):457–469
Yuan HW, Zhao L, Chen JJ, Yang Y, Xu DB, Tao SC, Zheng S, Shen YR, He Y, Shen CJ, Yan DL, Zheng BS (2018) Identification and expression profiling of the Aux/IAA gene family in Chinese hickory (Carya cathayensis Sarg.) during the grafting process. Plant Physiol Biochem 127:55–63
Han XY, Xu XY, Fang DD, Zhang TZ, Guo WZ (2012) Cloning and expression analysis of novel Aux/IAA family genes in Gossypium hirsutum. Gene 503(1):83–91
Tiwari SB, Hagen G, Guilfoyle TJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16:533–543
Winkler M, Niemeyer M, Hellmuth A, Janitza P, Christ G, Samodelov SL, Wilde V, Majovsky P, Trujillo M, Zurbriggen MD (2017) Variation in auxin sensing guides AUX/IAA transcriptional repressor ubiquitylation and destruction. Nat Commun 8:15706
Acknowledgements
This study was supported by National Natural Science Foundation of China (31971695, 31901346, 31470683, 31270716 and 31070604); National Key Research and Development Program of China (2018YFD1000604, 2018YFD1000600); Key Project of Zhejiang Provincial Natural Science Foundation (LZ18C160001); Independent Research Project of State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University (ZY20180208, ZY20180308); Open Foundation of State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University (KF201708); Overseas Expertise Introduction Project for Discipline Innovation (111 Project D18008); Key Research and Development Program of Zhejiang Province (2018C02004); National High Technology Research and Development Program of China (863 Program) (2013AA102605); Fruit Innovation Team Project of Zhejiang Province (2016C02052-12); Key Agricultural New Varieties Breeding Projects founded by Zhejiang Province Science and Technology Department (2016C02052-13); Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholar (LR13C160001); Open Foundation of First-class Discipline of Forestry, Zhejiang Province (201703); The First-class General Financial Grant from the China Postdoctoral Science Foundation (2017M610377).
Author information
Authors and Affiliations
Contributions
BZ and DY conceived and designed the study. DX performed bioinformatics analysis and wrote the manuscript. YY and ST conducted the experiments. HY, YW and XW took care of the plant samples. AS contributed to analyzed the data and reviewed the manuscript. CS contributed to the bioinformatics analysis and preparation of figures and tables.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, D., Yang, Y., Tao, S. et al. Identification and expression analysis of auxin-responsive GH3 family genes in Chinese hickory (Carya cathayensis) during grafting. Mol Biol Rep 47, 4495–4506 (2020). https://doi.org/10.1007/s11033-020-05529-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05529-w