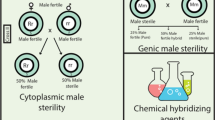
Abstract
Hybrid breeding can help us to meet the challenge of feeding a growing world population with limited agricultural land. The demand for soybean is expected to grow; however, the hybrid soybean is still in the process of commercialization even though considerable progress has been made in soybean genome and genetic studies in recent years. Here, we summarize recent advances in male sterility-based breeding programs and the current status of hybrid soybean breeding. A number of male-sterile lines with cytoplasmic male sterility (CMS), genic-controlled photoperiod/thermo-sensitive male sterility, and stable nuclear male sterility (GMS) have been identified in soybean. More than 40 hybrid soybean varieties have been bred using the CMS three-line hybrid system and the cultivation of hybrid soybean is still under way. The key to accelerating hybrid soybean breeding is to increase the out-crossing rate in an economical way. This review outlines current problems with the hybrid soybean breeding systems and explores the current efforts to make the hybrid soybean a commercial success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The area of soybean (Glycine max) cultivation in the world has expanded by more than 900% since the 1960s in North and South America, due to its significant roles in animal feeding and human nutrition (http://www.fao.org/faostat/en/#home). However, soybean yield per unit area has not changed significantly compared with rice, wheat, and maize, suggesting the lack of a true Green Revolution in soybean breeding (Liu, et al. 2020a). Soybean yield is determined by both the total number of nodes and the number of pods per node, therefore the yield increase cannot be achieved in soybean by simply adoption of the shorter varieties. There are several options to increase soybean yields, and hybrid breeding hold the greatest potential to boost yield.
Soybean is an autogamous legume species, and male sterility line is a prerequisites for commercially available hybrid breeding and large quantities of seed production. An earlier heterosis test demonstrated that significant yield increases could be achieved in soybean; the heterozygous F1 plants of 248 combinations yielded 20% more than their parental lines among the 1123 combinations that were tested (Sun, et al. 1999; Palmer, et al. 2001). However, hybrid breeding in soybean has received limited attention in contrast to maize and rice. Male-sterile female lines with cytoplasmic male sterility (CMS) or genic-controlled photoperiod/thermo-sensitive male sterility (P/TGMS) have been extensively used for many years in maize and rice (Chen and Liu, 2014; Wan, et al. 2019). Hybrid rice in China covers 50–60% of the total rice cultivation fields, which contributed greatly to rice yield and ensure food security (Kim and Zhang, 2018; Liao, et al. 2021).
Male sterility lines are available in soybean, and the first soybean CMS line was reported under the US patents No. 4545146 in Davis (1985). Since then, no further information on this CMS line has been reported. Considerable research on soybean CMS lines has been conducted in China since the early 80s of the last century (Sun, et al. 1994a; Palmer, et al. 2001). To date, more than 40 hybrid soybean varieties have been bred and approved in China after several generations of researchers with more than 40 years of efforts, and more than 30 invention patents and technical standards have been authorized for the use of new technologies and methods for soybean hybrid breeding (Sun, et al. 2021). However, the CMS genes and underlying molecular mechanisms are still unknown in soybean, which has restricted the development of commercial varieties.
With the explosion in genomic resources and the rapid development of molecular biology and technology, the biotechnology-based male-sterility (BMS) systems for hybrid breeding have been established in maize, rice and other crops and vegetables (Chang, et al. 2016; Wu, et al. 2016; Singh, et al. 2019). The BMS systems utilize nuclear male sterility to propagate the pure nuclear male sterile seeds on a large scale, which not only make the climate change not a threat to the pure hybrid seed production anymore, but also unlock the potential for breeding superior hybrids through expanding the parental germplasm pool. In soybean, the nuclear male sterile mutants ms4, ms1, ms6, and ms3 have been cloned in recent years (Thu, et al. 2019; Fang, et al. 2021; Jiang, et al. 2021; Nadeem, et al. 2021; Yu, et al. 2021; Hou, et al. 2022). To speed up the large-scale commercial cultivation of hybrid soybean, it is time to consider where to put the investments, should we continue to count on the three-line hybrid system and looking for the ideal maintainer and restorer lines, or we can rely on the BMS systems to realize the commercialization of hybrid soybean. In this review, we try to cover recent advances in cytoplasmic-nuclear and nuclear male sterility systems in soybean to see if the technological breakthroughs will make us to succeed in hybrid soybean production.
Male sterility in plant
Plant male sterility (MS) refers to the phenomenon that the stamen develops unnormal, losing the ability to produce the functionally active male gametes for fertilization. According to their phenotypic characteristics, Kaul (1988) divide MS into three categories including structural, sporogenous, and functional. Structural MS indicates that the stamen is either completely absent or abnormally formed, which results in the absence of pollen. Sporogenous MS indicates that the stamen is essentially morphological normal, but fail to produce functional microspores or pollen due to the failure of early microsporogenesis and late microgametogenesis. Functional MS indicates that the viable pollen is produced, but either cannot be released from the anther due to the absence of dehiscence or is unable to geminate on the stigma and to initiate fertilization.
On the other scheme, according to the origin of inheritance, two types of MS are distinguished: cytoplasmic male sterility (CMS) and nuclear or genic male sterility (GMS). CMS is co-controlled by the nuclear and cytoplasmic genes, while GMS is controlled by the nuclear genes alone. CMS is widely spread in the higher plants and more than 300 species possess CMS were reported up to now (Liu, et al. 2001). The CMS is the result of the incompatibility between nuclear and mitochondrial gene products and there are several ways to generate CMS, including wide/inter-specific hybridization, protoplasmic fusion, induced mutations and genetic engineering (Bohra, et al. 2016). GMS is derived from the changes in the structure and function of nuclear genes, most of which are caused by natural variation and can also be achieved by physical and chemical mutagenesis. Mostly, the fertility of a GMS line is controlled by a recessive gene, and rarely by a dominant gene.
Cytoplasmic male sterility (CMS) system
CMS/Rf (restorer-of-fertility) system, also known as the three-line hybrid system, comprises a cytoplasmic male sterile line, a maintainer line, and a restorer line. The sterile line contains a cytoplasmic male sterile gene, while lacks a nuclear restorer gene (Schnable and Wise, 1998), which is characterized by sterile pollen and unable to produce progeny by self-inbreeding. The maintainer line excludes the nuclear restorer gene but contains the fertile cytoplasmic gene (Chen and Liu, 2014). However, the restorer line preserves a functionally nuclear gene and with or without a fertile cytoplasmic gene (Chen and Liu, 2014). The pollens of both the maintainer and the restorer lines are fertile, so they can propagate by self-pollination. When the sterile line is used as the female parent, it can receive pollen from either the maintainer or restorer line and produce hybrid progeny. The maintainer line is used to cross with the male sterile line to reproduce the male sterile line, while the restorer line is used to cross with the male sterile line to produce hybrid progenies with heterosis to realize yield increase.
The CMS/Rf system has been exploited for hybrid seed production in plenty of crops such as maize, rice, wheat, rape, soybean, sorghum, carrot, sugar beet, sunflower, cotton, pepper, and petunia (Garcia, et al. 2019). Although this system has been successfully applied to soybean, the yield increase is still far away from that of rice and maize. One of the main reasons is the limited number of identified CMS lines, which heavily restricts the utilization of the three-line system in soybean hybrid seed production. In order to address this issue, the various cytoplasmic genes that produce MS phenotypes, along with their corresponding nuclear-encoded restorer-of-fertility genes, need to be identified urgently.
Genic male sterility (GMS) system
GMS is controlled by nuclear genes without the influence of the cytoplasmic genome that are either insensitive or sensitive to environmental conditions, called genetically stable GMS and environment-sensitive genic male sterility (EGMS), respectively. In the case of EGMS, male fertility is often impressionable to different environmental conditions, including photoperiod (PGMS), temperature (TGMS), photoperiod and temperature (PTGMS), and humidity (HGMS) (Chen and Liu, 2014; Xue, et al. 2018; Abbas, et al. 2021). EGMS is regarded as an efficient genetic tool to develop two-line hybrids, since the need of a maintainer line can be eliminated, and the male sterile line can be propagated by self-pollination under specific conditions (Garcia, et al. 2019). In this system, almost every conventional inbred line is able to restore the fertility of the male-sterile line, and no negative effects related to sterility-inducing cytoplasm have been observed. Furthermore, genes of this system can be easily transferred to other genetic backgrounds (Yu, et al. 2016) developed new “transgene clean” commercial TGMS lines in rice by knocking out TMS5 via CRISPR/Cas9. Whereafter, Li et al. (2017) produced TGMS maize by targeted mutation of the maize homolog of rice TMS5 (called ZmTMS5) using the CRISPR/Cas9 editing system. In addition, two rice reverse PGMS lines in japonica cultivars 9522 and JY5B were also generated by editing Carbon Starved Anther (CSA) gene using CRISPR/Cas9 (Li, et al. 2016). Furthermore, Qi et al. (2012; Wang, et al. 2016; Dai, et al. 2022). To narrow down the candidate region for Rf1 gene for CMS-RN, Guo et al. (2022) constructed an F2 population by crossing JLCMS204A with JLR230 (restorer line), and the gene was located between the marker dCAPS-1 and BARCSOYSSR_16_1076., A recent study identified Glyma.16G161900 as the candidate gene of Rf1 (Yang, et al. 2023). In addition, Glyma.09G171200, encoding a pentatricopeptide repeat (PRR) protein, was confirmed as the candidate gene of another Rf3 gene for CMS-RN (Sun, et al. 2022). In addition, the Rf gene of CMS-ZD type was located to the marker BARCSOYSSR_16_1064 and BARCSOYSSR_16_1082 on Chr. 16 (Dong, et al. 2012). Another Rf-m gene of CMS-ZD allocating on Chr. 16 was identified between GmSSR1602 and GmSSR1610 (Wang, et al. 2016). Furthermore, another PPR gene (GmPPR576, Glyma.16G161900) was identified as the candidate Rf gene of CMS-N8855 type (Wang, et al. 2021), which was consistent with that of Rf1 gene (Yang, et al. 2023). Four Rf genes for CMS-RN, CMS-ZD and CMS-N8855 were closely distributed on Chr. 16 with a close region (Dong, et al. 2012; Wang, et al. 2016; Wang, et al. 2021; Guo, et al. 2022), whether they were controlled by the same gene needs to be further verified.
In addition, due to the lack of systematically cytological observation and the inconsistent cytological phenotype even for the same CMS type, for example, the CMS-N8855 line, whether the different CMS types are really distinguished from each other should be confirmed (Ding, et al. 2001; Fan 2003). Furthermore, an unusual phenomenon also happened that the same maintainer and restorer line can maintain and restore different CMS type, viz. YA (CMS-RN) and ZA (CMS-ZD) (Zhao, et al. 1998). Considering the contradictions, we cannot rule out the possibility that the six classified CMS types may not be completely different from each other.
GMS system in soybean
The first report of GMS line in soybean was published in 1928, the mutant st1 was both male and female sterile caused by abnormal chromosome association, which was controlled by a single recessive gene (Owen 1928). To date, approximately 30 GMS lines have been identified in soybean (Table 3). According to the phenotypic characteristics, fs1fs2 (Johns and Palmer, 1982) and ft (transformed flower) (Singh and Jha, 1978) belong to the structural MS, the others belong to sporogenous MS, and no functional MS has been reported in soybean.
Two PGMS including ms3 (Chaudhari and Davis, 1977) and 88-428-BY (Wei 1991) and three TGMS including ms8 (Palmer 2000), ms9 (Palmer 2000), and msp (Stelly and Palmer, 1980) have already been reported. In addition, st1-st8, NJS-1H, D8804-7, and fs1fs2 mutants were both male and female sterile (Owen 1928; Hadley and Starnes, 1964; Palmer 1974; Johns and Palmer, 1982; Palmer and Kaul, 1983; Skorupska and Palmer, 1990; Zhao, et al. 1995; Ilarsian, et al. 1997; Palmer and Horner, 2000; Kato and Palmer, 2003; Li, et al. 2010; Speth, et al. 2015). The ms1 was the first GMS line that showed male sterile and female fertile phenotype in soybean (Brim and Young, 1971). In addition, ms2, ms4-ms7, ms12, MJ89-1, msMOS, msNJ, N7241S, Wh921, mst-M, and ft also belong to the male sterile and female fertile category (Singh and Jha, 1978; Palmer 1979; Buss 1983; Graybosch, et al. 1984; Graybosch and Palmer, 1985; Skorupska and Palmer, 1989; Ma, et al. 1993; **, et al. 1997; Zhang, et al. 1999b; Palmer 2000; Zhao, et al. 2005; Zhang 2019; Zhao, et al. 2019).
Although five PGMS and TGMS lines have been identified, so far, only MS3 (Glyma.02G107600), encoding a plant homeodomain (PHD) protein, has been identified (Hou, et al. 2022). The fertility of mutant ms3 mutant can restore under long-day conditions, thus the mutant could be used to create a new, more stable photoperiod-sensitive genic male sterility line for two-line hybrid seed production in soybean. With the rapid development of BMS systems in rice and maize, more and more attempts have been made in soybean. The 13 GMS lines (ms1, ms2, ms4-ms7, ms12, NJ89-1, msMOS, msNJ, N7241S, Wh921, and mst-M) displaying male sterile and female fertile phenotypes are suitable for exploiting this new technology in soybean. In order to make this design a reality, a large number of of works have been performed to explore the candidate sterile genes for these GMS mutants. MS4 (Glyma.02G243200) is the first GMS gene that has been discovered in soybean by fine map**, which encodes a MALE MEIOCYTE DEATH 1 (MMD1) protein (Thu, et al. 2019). The functional confirmation of MS4 in regulating male fertility was conducted by heterologous expression in Arabidopsis mmd1 mutant (Thu, et al. 2019). Subsequently, the function of MS12 (Glyma.10G117000) was also confirmed by QTL map** and functional complementation of soybean gene in Arabidopsis cdc20.2 (cell division cycle 20.2) mutant (Zhang 2019). In 2021, both MS1 (Glyma.13G114200) and MS6 (Glyma.13G066600) have been identified by fine map** (Fang, et al. 2021; Jiang, et al. 2021; Nadeem, et al. 2021; Yu, et al. 2021). MS6 encodes a Tapetal Development and Functional 1 (TDF1) protein, a R2R3 MYB transcription factor, and predominantly expressed in anther, where it regulated the formation of pollen grain (Yu, et al. 2021). MS1, encoding a NPK1-ACTIVATING KINESIN 2 (NACK2) protein, is essential for cell plate formation after cytokinesis by directly control of the phragmoplast expansion (Fang, et al. 2021). Identification and characterization of GMS genes will provide more options for building the BMS systems for hybrid soybean production.
Challenges and prospects in the commercialization of hybrid soybean
Although more than 40 hybrid soybean varieties have been generated from the three-line hybrid system (cytoplasmic male sterility), the unstable sterility of MS line and the high cost of hybrid seed production constrained the large-scale application of heterosis in soybean, which makes the cultivation of hybrid soybean still has a long way to go. We believe that the three components are the keys to make hybrid soybean a commercial success:
Identify the male sterile lines with high out-crossing rate
The out-crossing rate is the key determinant of hybrid seed production. Seed production has not been efficient and cost-effective for hybrid soybean. The main reason is that the mutations in causing the male sterility also very often have pleiotropic effects and lead to the defect in female function, which make the male sterile lines with low seed set. The identification of ms1 locus revealed that the gene was highly expressed in style and ovary and may also function in megagametogenesis or embryo development in soybean (Fang, et al. 2021). Studies had focused on the outcrossing rate on male sterile plants, the most promising record was the ms2 mutant, the outcrossing rate on male sterile plants was 74% of the self-pollinated plants (Carter, et al. 1986; Perez, et al. 2009). The feasible solution is to speed up the cloning of the causal gene for male sterile mutants that have good recorded with seed-set, and simultaneously generate new male sterile lines by genome editing to make the mutation only affect the male fertility and without any effects on female productivity and other growth habits.
Besides the finding of ideal male sterility lines from the genetic perspective, the structure changes of flower and reproductive organs, for examples, the stigma protruding beyond the anthers, more pollen grains, and nectaries produce more fluids and/or volatiles, could increase the opportunity for cross-pollination (Palmer, et al. 2001). Pollen grain from soybean is heavy and sticky and the insect-mediated pollination is still indispensable even when the soybean flower is opened. The improvement of techniques for hybrid seed production is equally important for the commercialization of hybrid soybean, including the management of insect pollinators for cross-pollination and the suitable environment for both pollinators and soybeans, etc (Palmer, et al. 2001; Garibaldi, et al. 2021).
Incorporate genomic selection to precise guidance on hybridization combination
Breeding 4.0 has been considered the next revolution of maize breeding (Wallace, et al. 2018). Even though the soybean breeding program is still at the Breeding 2.0 to 3.0 stages with molecular markers and genomic data to complement phenotypic data, the high-quality graph-based soybean pan-genome and the low cost of genome sequencing will turn promise into practice (Liu, et al. 2020b). The genotypes of soybean germplasm lines will be collected using high-throughput genoty** approaches such as next-generation sequencing (NGS) and SNP array platforms. Genetic variations among soybean germplasm of different origins/sources will make the selection of superior hybrid cost-effective.
Good understanding of the molecular mechanisms of anther development in soybean
Little is known about the biological processes and genes that regulate anther and pollen development in soybean. Like most (70%) angiosperms, soybean produces bicellular pollen. By contrast, rice and Arabidopsis both produce tricellular pollen, the biological significance of the evolution of these two types of pollen grains is still unclear (Williams, et al. 2014). Bicellular pollen undergoes mitotic division to form two sperm cells after germination; prior to anthesis, tricellular pollen forms a male germ unit (MGU) that develops rapidly, which may make tricellular pollen favored in angiosperms that demand rapid reproduction (Hackenberg and Twell, 2019). So, the knowledge of extensive studies of anther and pollen formation in Arabidopsis and rice should not be simply transferred to soybean. Taking advantage of the comparative transcriptome analysis, the uncovering of anther-specific genes, genetic networks, and hub genes in soybean anther development will provide important insights into the molecular events underlying soybean reproductive developmental processes, as well as valuable resources for the plant reproductive biology community in the areas of pollen evolution, pollination/fertilization, and hybrid breeding.
In summary, on-going and future research should consider the enhancement of hybrid seed production efficiency, and the long-term investment and commitment will definitely make the commercialization of hybrid soybean a reality.
Data availability
The tables are included in this article albertsen.
References
Abbas A, Yu P, Sun LP, Yang ZF, Chen DB, Cheng SH, Cao LY (2021) Exploiting genic male sterility in rice: from molecular dissection to breeding applications. Front Plant Sci 12:629314
Albertsen MC, Palmer RG (1979) A comparative light-and electron-microscopic study of microsporogenesis in male sterile (MS) and male fertile soybeans (Glycine max (L.) Merr.). Am J Bot 66:253–265
An XL, Dong Z, Tian YH, **e K, Wu SW, Zhu TT, Zhang DF, Zhou Y, Niu CF, Ma B, Hou QC, Bao JX, Zhang SM, Li ZW, Wang YB, Yan TW, Sun XJ, Zhang YW, Li JP, Wan XY (2019) ZmMs30 encoding a novel GDSL lipase is essential for male fertility and valuable for hybrid breeding in maize. Mol Plant 12:343–359
Bai ZY, Ding XL, Zhang RJ, Yang YH, Wei BG, Yang SP, Gai JY (2022) Transcriptome analysis reveals the genes related to pollen abortion in a cytoplasmic male-sterile soybean (Glycine max (L.) Merr.). Int J Mol Sci 23:12227
Bohra A, Jha UC, Adhimoolam P, Bisht D, Singh NP (2016) Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep 35:967–993
Brim CA, Young MF (1971) Inheritance of a male-sterile character in soybeans. Crop Sci 11:564–566
Buss GR (1983) Inheritance of a male-sterile mutant from irradiated Essex soybean. Soybean Genet Newsl 10:104–108
Carter TE, Brar JG, Burton JW, Fonseca AL (1986) Seed yield on field-grown ms2 ms2 male-sterile plants. Soybean Genet Newsl 13:159–163
Cervantes-Martinez I, Sandhu D, Xu M, Ortiz-Pérez E, Kato KK, Horner HT, Palmer RG (2009) The male sterility locus ms3 is present in a fertility controlling gene cluster in soybean. J Hered 100:565–570
Cervantes-Martinez I, Xu M, Zhang L, Huang Z, Kato KK, Horner HT, Palmer RG (2007) Molecular map** of male-sterility loci ms2 and ms9 in soybean. Crop Sci 47:374–379
Chang ZY, Chen ZF, Wang N, **e G, Lu JW, Yan W, Zhou JL, Tang XY, Deng XW (2016) Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc Natl Acad Sci U S A 113:14145–14150
Chaudhari HK, Davis WH (1977) A new male-sterile strain in Wabash soybeans. J Hered 68:266–267
Chen LT, Liu YG (2014) Male sterility and fertility restoration in crops. Annu Rev Plant Biol 65:579–606
Chen X, Yang SX, Zhang YH, Zhu XB, Yang XJ, Zhang CB, Li HY, Feng XZ (2021) Generation of male-sterile soybean lines with the CRISPR/Cas9 system. Crop J 9:1270–1277
Dai JY, Zhang RJ, Wei BG, Nie ZX, **ng GN, Zhao TJ, Yang SP, Gai JY (2017) Key biological factors related to outcrossing-productivity of cytoplasmic-nuclear male-sterile lines in soybean [Glycine max (L.) Merr.]. Euphytica 213:1–18
Davis WH. 1985. Route to hybrid soybean production. US Patent 4545146.
Delannay X, Palmer RG (1982) Genetics and cytology of the ms4 male-sterile soybean. J Hered 73:219–223
Deng HF, Shu FB, Yuan DY (1999) An overview of research and utilization of Annong S-1. Hybrid Rice 14:1–3
Ding DR, Gai JY, Cui ZL, Qiu JX (2001) Cytological studies on pollen abortion in cytoplasmic-nuclear male sterile soybean line NJCMS1A. Soybean Sci 20:167–171
Ding DR, Gai JY, Cui ZL, Qiu JX (2002) Development of a cytoplasmic-nuclear male-sterile line of soybean. Euphytica 124:85–91
Dong DK, Li Z, Yuan FJ, Zhu SL, Chen P, Yu W, Yang QH, Fu XJ, Yu XM, Li BQ (2012) Inheritance and fine map** of a restorer-of-fertility (Rf) gene for the cytoplasmic male sterility in soybean. Plant Sci 188:36–40
Fan JM (2003) Studies on cyto-morphological and cyto-chemical features of cytoplasmic-nuclear male-sterile lines of soybeans (Glycine max (L.) Merr.). Master’s Dissertation: Nan**g Agricultural University
Fang XL, Sun XY, Yang XD, Li Q, Lin CJ, Xu J, Gong WJ, Wang YF, Liu L, Zhao LM, Liu BH, Qin J, Zhang MC, Zhang CB, Kong FJ, Li MN (2021) MS1 is essential for male fertility by regulating the microsporocyte cell plate expansion in soybean. Sci China Life Sci 64:1533–1545
Fox T, DeBruin J, Haug Collet K, Trimnell M, Clapp J, Leonard A, Li B, Scolaro E, Collinson S, Glassman K, Miller M, Schussler J, Dolan D, Liu L, Gho C, Albertsen M, Loussaert D, Shen B (2017) A single point mutation in Ms44 results in dominant male sterility and improves nitrogen use efficiency in maize. Plant Biotechnol J 15:942–952
Frasch RM, Weigand C, Perez PT, Palmer RG, Sandhu D (2011) Molecular map** of 2 environmentally sensitive male-sterile mutants in soybean. J Hered 102:11–16
Gai JY, Cui ZL, Ji DF, Ren ZJ, Ding DR (1995) A report on the nuclear cytoplasmic male sterility from a cross between two soybean cultivars. Soybean Genet Newsl 22:55–58
Garcia LE, Edera AA, Marfil CF, Sanchez-Puerta M (2019) Male sterility and somatic hybridization in plant breeding. Rev Fac Cienc Agrar, Univ Nac Cuyo 51:475–486
Garibaldi LA, Schulte LA, Jodar DNN, Carella DSG, Kremen C (2021) Time to integrate pollinator science into soybean production. Trends Ecol Evol 36:573–575
Graybosch RA, Bernard RL, Cremeens CR, Palmer RG (1984) Genetic and cytological studies of a male-sterile, female-fertile soybean mutant: a new male-sterile gene (ms2) in Glycine max (L.) Merr. J Hered 75:383–388
Graybosch RA, Palmer RG (1985) Male sterility in soybean (Glycine max). I. Phenotypic expression of the ms2 mutant. Am J Bot 72:1738–1750
Guo FL, Lin CJ, Wang PN, Yang XL, Wu Z, Peng B, Zhao LM, Zhang CB (2022) Fine map** of a restorer-of-fertility gene GmRf1 for the cytoplasmic male sterility in soybean. J Plant Genet Resour 23:518–526
Hackenberg D, Twell D (2019) The evolution and patterning of male gametophyte development. Curr Top Dev Biol 131:257–298
Hadley HH, Starnes WJ (1964) Sterility in soybeans caused by asynapsis1. Crop Sci 4:421–424
Hou JJ, Fan WW, Ma RR, Li B, Yuan ZH, Huang WX, Wu YL, Hu Q, Lin CJ, Zhao XQ, Peng B, Zhao LM, Zhang CB, Sun LJ (2022) MALE STERILITY 3 encodes a plant homeodomain-finger protein for male fertility in soybean. J Integr Plant Biol 64:1076–1086
Ilarsian H, Skorupska HT, Horner HT, Palmer RG (1997) Cytology and genetics of a tissue culture-derived soybean genic male-sterile, female-sterile. J Hered 88:129–138
Ilarslan H, Horner HT, Palmer R (1999) Genetics and cytology of a new male-sterile, female-fertile soybean mutant. Crop Sci 39:58–64
Jiang BJ, Chen L, Yang CY, Wu TT, Yuan S, Wu CX, Zhang MC, Gai JY, Han TF, Hou WS, Sun S (2021) The cloning and CRISPR/Cas9-mediated mutagenesis of a male sterility gene MS1 of soybean. Plant Biotechnol J 19:1098–1100
** W, Horner HT, Palmer RG (1997) Genetics and cytology of a new genic male-sterile soybean [Glycine max (L.) Merr.]. Sex Plant Reprod 10:13–21
Johns CW, Palmer RG (1982) Floral development of a flower-structure mutant in soybeans, Glycine max (L.) Merr.(Leguminosae). Am J Bot 69:829–842
Kato KK, Palmer RG (2003) Molecular map** of the male-sterile, female-sterile mutant gene (st8) in soybean. J Hered 94:425–428
Kaul MLH (1988) Male sterility in higher plants. Springer-Verlag, New York
Kim YJ, Zhang DB (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci 23:53–65
Li J, Zhang HW, Si XM, Tian YH, Chen KL, Liu JX, Chen HB, Gao CX (2017) Generation of thermosensitive male-sterile maize by targeted knockout of the ZmTMS5 gene. J Genet Genomics 44:465–468
Li JJ, Nadeem M, Sun GL, Wang XB, Qiu LJ (2019) Male sterility in soybean: occurrence, molecular basis and utilization. Plant Breed 138:659–676
Li QL, Zhang DB, Chen MJ, Liang WQ, Wei JJ, Qi YP, Yuan Z (2016) Development of japonica photo-sensitive genic male sterile rice lines by editing carbon starved anther using CRISPR/Cas9. J Genet Genomics 43:415–419
Li SG, Zhao TJ, Gai JY (2010) Cytological and genetical characterization of a nuclear male-sterile soybean mutant NJS-1H. Soybean Sci 29:181–185
Liao CC, Yan W, Chen ZF, **e G, Deng XW, Tang XY (2021) Innovation and development of the third-generation hybrid rice technology. Crop J 9:693–701
Lin CJ, Peng B, Li YK, Wang PN, Zhao GL, Ding XY, Li R, Zhao LM, Zhang CB (2020) Cytoplasm types affect DNA methylation among different cytoplasmic male sterility lines and their maintainer line in soybean (Glycine max L.). Plants 9:385
Lin CJ, Zhang CB, Zhao HK, **ng SC, Wang YM, Liu XD, Yuan CP, Zhao LM, Dong YS (2014) Sequencing of the chloroplast genomes of cytoplasmic male-sterile and male-fertile lines of soybean and identification of polymorphic markers. Plant Sci 229:208–214
Liu SL, Zhang M, Feng F, Tian ZX (2020a) Toward a “green revolution” for soybean. Mol Plant 13:688–697
Liu YC, Du HL, Li PC, Shen YT, Peng H, Liu SL, Zhou GA, Zhang HK, Liu Z, Shi M, Huang XH, L i Y, Zhang M, Wang Z, Zhu BG, Han B, Liang CZ, Tian ZX. (2020b) Pan-genome of wild and cultivated soybeans. Cell 182:162–176
Liu ZS, Guan CY, Chen SY (2001) Application and research on mechanism of plant male sterility. Press of Chinese Agriculture, Bei**g
Ma GR, Liu YB, Gai JY (1993) NJ89-1, a new male sterile mutant of soybean. Soybean Sci 12:172–174
Nadeem M, Chen AD, Hong HL, Li DD, Li JJ, Zhao D, Wang W, Wang XB, Qiu LJ (2021) GmMs1 encodes a kinesin-like protein essential for male fertility in soybean (Glycine max L.). J Integr Plant Biol 63:1054–1064
Nie ZX, Zhao TJ, Liu MF, Dai JY, He TT, Lyu D, Zhao JM, Yang SP, Gai JY (2019) Molecular map** of a novel male-sterile gene msNJ in soybean [Glycine max (L.) Merr.]. Plant Reprod 32:371–380
Nie ZX, Zhao TJ, Yang SP, Gai JY (2017) Development of a cytoplasmic male-sterile line NJCMS 4A for hybrid soybean production. Plant Breed 136:516–525
Owen FV (1928) A sterile character in soybeans. Plant Physiol 3:223–226
Palmer RG (1974) A desynaptic mutant in the soybean. J Hered 65:280–286
Palmer RG (1979) Inheritance of male-sterile, female-fertile mutant ms4. Soybean Genet Newsl 6:63–64
Palmer RG (2000) Genetics of four male-sterile, female-fertile soybean mutants. Crop Sci 40:78–83
Palmer RG, Gai JY, Sun H, Burton JW (2001) Production and evaluation of hybrid soybean. Plant Breed Rev 21:263–307
Palmer RG, Horner HT (2000) Genetics and cytology of a genic male-sterile, female-sterile mutant from a transposon-containing soybean population. J Hered 91:378–383
Palmer RG, Johns CW, Muir PS (1980) Genetics and cytology of the ms3 male-sterile soybean. J Hered 71:343–348
Palmer RG, Kaul MLH (1983) Genetics, cytology, and linkage studies of a desynaptic soybean mutant. J Hered 74:260–264
Palmer RG, Winger CL, Albertsen MC (1978) Four independent mutations at the ms1 locus in soybeans. Crop Sci 18:727–729
Peng YH, Yang G, Yuan J (1994) World Soybean Research Conference V. Genetic analysis of a new type of male sterile soybean. Funny Publishing Limited Partnership, Bangkok, Thailand
Perez PT, Cianzio SR, Palmer RG (2009) Evaluation of soybean [Glycine max (L.) Merr.] F1 hybrids. J Crop Improv 23:1–18
Qi XT, Zhang CS, Zhu JJ, Liu CL, Huang CL, Li XH, **e CX (2020) Genome editing enables next-generation hybrid seed production technology. Mol Plant 13:1262–1269
Ren C (2005) Studied on soybean cytoplasmic-nuclear sterile line W931A in cytological characteristics of abortion and its heterosis utilization. Master’s Dissertation: Anhui Agricultural University
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Shi MS (1985) The discovery and study of the photosensitive recessive male-sterile rice (Oryza sativa L. ssp. japonica). Sci Agri Sin 2:44–48
Singh BB, Jha AN (1978) Abnormal differentiation of floral parts in a mutant strain of soybean. J Hered 69:143–144
Singh S, Dey SS, Bhatia R, Kumar R, Behera TK (2019) Current understanding of male sterility systems in vegetable Brassicas and their exploitation in hybrid breeding. Plant Reprod 32:231–256
Skorupska H, Palmer RG (1989) Genetics and cytology of the ms6 male-sterile soybean. J Hered 80:304–310
Skorupska HT, Palmer RG (1990) Additional sterile mutations in soybean, Glycine max (L.) Merr. J Eur Econ Assoc 81:296–300
Song SF, Wang TK, Li YX, Hu J, Kan RF, Qiu MD, Deng YD, Liu PX, Zhang LC, Dong H, Li CX, Yu D, Li XQ, Yuan DY, Yuan LP, Li L (2021) A novel strategy for creating a new system of third-generation hybrid rice technology using a cytoplasmic sterility gene and a genic male-sterile gene. Plant Biotechnol J 19:251–260
Speth B, Rogers JP, Boonyoo N, VanMeter AJ, Baumbach J, Ott A, Moore J, Cina T, Palmer RG, Sandhu D (2015) Molecular map** of five soybean genes involved in male-sterility, female-sterility. Genome 58:143–149
Stelly DM, Palmer RG (1980) A partially male-sterile mutant line of soybeans, Glycine max (L.) Merr.: characterization of the msp phenotype variation. Euphytica 29:539–546
Sun H, Zhao LM, Huang M (1994a) Study on cytoplasmic-nucleic male sterile soybean. Chin Sci Bull 38:1535–1536
Sun H, Zhao LM, Huang M (1994b) Cytoplasmic-nuclear male sterile soybean line from interspecific crosses between G. max and G. soja. In: Proceedings World Soybean Research Conference V: Soybean Feeds the World, Chiang Mai, Thailand
Sun H, Zhao LM, Huang M. 2001. Cytoplasmic-genetic male sterile soybean and method for producing hybrid soybean. In. US Patent 6320098B1.
Sun H, Zhao LM, Li J, Wang S (1999) World Soybean Research Conference VI. The investigation of heterosis and pollen transfer in soybean, Champaign, Illinois
Sun H, Zhao LM, Wang SM, Wang YQ, Li JP (2003) Progress on the utilization of heterosis in soybean. Chin J Oil Crop Sci 25:92–96
Sun YY, Zhang Y, Jia SG, Lin CJ, Zhang JY, Yan H, Peng B, Zhao LM, Zhang W, Zhang CB (2022) Identification of a candidate restorer-of-fertility gene Rf3 encoding a pentatricopeptide repeat protein for the cytoplasmic male sterility in soybean. Int J Mol Sci 23:5388
Sun YY, Zhao LM, Zhang W, Zhang CB (2021) Research process on utilization of soybean heterosis. Soybean Sci Tech 6:26–35
Thu SW, Rai KM, Sandhu D, Rajangam A, Balasubramanian VK, Palmer RG, Mendu V (2019) Mutation in a PHD-finger protein MS4 causes male sterility in soybean. BMC Plant Biol 19:1–12
Wallace JG, Rodgers-Melnick E, Buckler ES (2018) On the road to breeding 4.0: unraveling the good, the bad, and the boring of crop quantitative genomics. Annu Rev Genet 52:421–444
Wan XY, Wu SW, Li ZW, Dong ZY, An XL, Ma B, Tian YH, Li JP (2019) Maize genic male-sterility genes and their applications in hybrid breeding: progress and perspectives. Mol Plant 12:321–342
Wang DG, Zhang L, Li JK, Hu GY, Wu Q, Jiang HY, Huang ZP (2016) The restorer gene for soybean M-type cytoplasmic male sterility, Rf-m, is located in a PPR gene-rich region on chromosome 16. Plant Breed 135:342–348
Wang F, Wei B, Li G, Li Y (2004) A cytological observation of the pollen mother cells of the photoperiod-sensitive male sterile soybean plant of 88-428BY-827. Sci Agri Sin 37:1110–1113
Wang TL, He TT, Ding XL, Zhang QQ, Yang LS, Nie ZX, Zhao TJ, Gai JY, Yang SP (2021) Confirmation of GmPPR576 as a fertility restorer gene of cytoplasmic male sterility in soybean. J Exp Bot 72:7729–7742
Wang Y, Zhao LM, Wang X, Sun H (2010) Molecular map** of a fertility restorer gene for cytoplasmic male sterility in soybean. Plant Breed 129:9–12
Wei BG (1991) Preliminary study on the discovery of photoperiod sensitive male sterile line in soybean. Crop Var Resour 3:12
Williams JH, Taylor ML, O'Meara BC (2014) Repeated evolution of tricellular (and bicellular) pollen. Am J Bot 101:559–571
Wu YZ, Fox TW, Trimnell MR, Wang LJ, Xu R, Cigan AM, Huffman GA, Garnaat CW, Hershey H, Albertsen MC (2016) Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnol J 14:1046–1054
Xue ZY, Xu X, Zhou Y, Wang XN, Zhang YC, Liu D, Zhao BB, Duan LX, Qi XQ (2018) Deficiency of a triterpene pathway results in humidity-sensitive genic male sterility in rice. Nat Commun 9:1–10
Yan H, Zhang JY, Zhang CB, Peng B, Zhang WL, Wang PN, Ding XY, Liu BH, Feng XZ, Zhao LM (2019) Genetic effects and plant architecture influences on outcrossing rate in soybean. J Integr Agr 18:1971–1979
Yang SP, Gai JY, Qiu JX (2003) Allelism tests of the male sterile gene of the mutant NJ89-1 in soybeans. Acta Agron Sin 29:372–378
Yang XL, Guo FL, Gao MM, Zhang ZD, Lin CJ, Sun YY, Zhang JY, Peng B, Zhao LM, Zhang CB (2023) Preliminary identification and functional marker development of a CMS restorer-of-fertility gene GmRf1 in soybean. J Plant Genet Resour 24:1185–1192
Yu JP, Zhao GL, Li W, Zhang Y, Wang P, Fu AG, Zhao LM, Zhang CB, Xu M (2021) A single nucleotide polymorphism in an R2R3 MYB transcription factor gene triggers the male sterility in soybean ms6 (Ames1). Theor Appl Genet 134:3661–3674
Yu YH, **ang M, Que BC, Wang Y, Zheng YJ, Liu Y, Li ZY, Chen GH, Tang L, Xu ZJ (2015) The relationship between indica and japonica composition of the parents of two-line japonica hybrid rice and yield and its components. Jiangsu Agric Sci 43:15–18
Zhang CB, Fu FY, Lin CJ, Ding XY, Zhang JY, Yan H, Wang PN, Zhang W, Peng B, Zhao LM (2021) MicroRNAs involved in regulatory cytoplasmic male sterility by analysis RNA-seq and small RNA-seq in soybean. Front Genet 12:654146
Zhang CB, Lin CJ, Xu ZR, Chen ZH, Peng B, Wang PN, Ding XY, Zhao LM (2018a) DNA methylation differences in soybean hybrids and their parental lines. Russ J Plant Physl 65:357–363
Zhang DF, Wu SW, An XL, **e K, Dong ZY, Zhou Y, Xu LW, Fang W, Liu SS, Liu SS, Zhu TT, Li JP, Rao LQ, Zhao JR, Wan XY (2018b) Construction of a multicontrol sterility system for a maize male-sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD-finger transcription factor. Plant Biotechnol J 16:459–471
Zhang JY, Sun H, Zhao LM, Zhang CB, Yan H, Peng B, Li WB (2018c) Nectar secretion of RN-type cytoplasmic male sterility three-lines in soybean [Glycine max (L.) Merr.]. J Integr Agr 17:1085–1092
Zhang L, Dai OH, Huang ZP, Li JK (1999a) Breeding and fertility performance of nuclear and plasmic interaction of M-type male sterility line in soybean. Sci Agri Sin 32:34–38
Zhang L, Dai OH, Huang ZP, Li JK, Zhang LY, Hu C (2007) Breeding of hybrid soybean Zayoudou No. 1. Soybean Bull 2:14–16
Zhang L, Huang ZP, Li JK, Dai OH (1999b) Preliminary study of male sterile mutant Wh921 and its heterosis in soybean. Chin J Oil Crop Sci 21:20–23
Zhang Y (2019) Clone and functional analysis of soybean male sterility gene. Master’s Dissertation: Harbin Normal University
Zhao LM, Liu DP, Sun H, Yun Y, Huang M (1995) A sterile material of soybean gained by introducing exogenous DNA. Soybean Sci 14:83–87
Zhao LM, Sun H, Huang M (1998) Breeding of ZA type cytoplasmic male sterile line and its preliminary study. Soybean Sci 17:268–270
Zhao LM, Sun H, Wang SM, Wang YQ, Huang M, Li JP (2004) Breeding of hybrid soybean HybSoy 1. Chin J Oil Crop Sci 26:15–17
Zhao QS, Tong Y, Yang CY, Yang YQ, Zhang MC (2019) Identification and map** of a new soybean male-sterile gene mst-M. Front Plant Sci 10:94
Zhao TJ, Gai JY (2006) Discovery of new male-sterile cytoplasm sources and development of a new cytoplasmic-nuclear male-sterile line NJCMS3A in soybean. Euphytica 152:387–396
Zhao TJ, Yang SP, Gai JY (2005) Discovery of a dominant nuclear male sterile mutant N7241S in soybean and analysis of its inheritance. Sci Agri Sin 38:22–26
Zhou H, He M, Li J, Chen L, Huang ZF, Zheng SY, Zhu LY, Ni ED, Jiang DG, Zhao BR (2016) Development of commercial thermo-sensitive genic male sterile rice accelerates hybrid rice breeding using the CRISPR/Cas9-mediated TMS5 editing system. Sci Rep 6:1–12
Funding
This work was funded by the National Science Foundation of China (31971969, 32072084 and 32201803), Science and Technology Development Plan Project of Jilin Province (20210302005NC) earmarked Fund for China Agriculture Research System (CARS-04) and the National Key Research and Development Program of China (grant no. 2021YFF1001201).
Author information
Authors and Affiliations
Contributions
M. Li and C. Zhang designed the writing outline of this review article. X Fang, Y. Sun, J. Li and M. Li wrote the manuscript. M. Li and C. Zhang revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors approved the submission.
Consent to participate
Not applicable.
Consent for publication
Yes.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Soybean Functional Genomics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, X., Sun, Y., Li, J. et al. Male sterility and hybrid breeding in soybean. Mol Breeding 43, 47 (2023). https://doi.org/10.1007/s11032-023-01390-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-023-01390-4