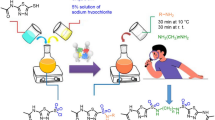
Abstract
The synthesis of novel, high-yield derivatives of chromenoazepine was investigated in this work. CuO/TiO2@MWCNTs was used as a nanocatalyst in a multicomponent reaction involving 4-aminocumarine, activated acetylenic chemicals, and alkyl bromide in room temperature water to create these novel compounds. Using MCRs of 4-aminocumarine, isothiocyanate, and alkyl bromide in the presence of CuO/TiO2@MWCNTs as nanocatalysts in room-temperature water, chromenothiazepines were synthesized under comparable conditions. The freshly synthesized azepine exhibits antioxidant activity since its NH group has undergone two evaluation processes. Additionally, using two types of Gram-negative bacteria in a disk distribution procedure, the antibacterial activity of recently developed azepines was evaluated, and these compounds also inhibited the growth of Gram-positive bacteria. This method’s benefits include quick reaction times, large product yields, and straightforward catalyst and product separation through easy steps.










Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the supplementary material of this article.
References
Reymond J-L, Awale M (2012) Exploring chemical space for drug discovery using the chemical universe database. ACS Chem Neurosci 3(9):649–657. https://doi.org/10.1021/cn3000422
James MJ, O’Brien P, Taylor RJK, Unsworth WP (2016) Synthesis of Spirocyclic Indolenines. Chem Eur J 22:2856. https://doi.org/10.1002/chem.201503835
Welsch ME, Snyder SA, Stockwell BR (2010) Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol 14:347–361. https://doi.org/10.1016/j.cbpa.2010.02.018
Kalaria PN, Karad SC, Raval DK (2018) A review on diverse heterocyclic compounds as the privileged scaffolds in antimalarial drug discovery. Eur J Med Chem 158:917–936. https://doi.org/10.1016/j.ejmech.2018.08.040
Desai N, Trivedi A, Pandit U, Dodiya A, Rao VK, Desai P (2016) Hybrid bioactive heterocycles as potential antimicrobial agents: a review. Mini Rev Med Chem 16(18):1500–1526. https://doi.org/10.2174/1389557516666160609075620
Fouad MM, El-Bendary ER, Suddek GM, Shehata IA, El-Kerdawy MM (2018) Synthesis and in vitro antitumor evaluation of some new thiophenes and thieno[2,3-d]pyrimidine derivatives. Bioorg Chem 81:587598. https://doi.org/10.1016/j.bioorg.2018.09.022
Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C, Baptista PV, Fernandes AR (2015) Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules 20:16852–16891. https://doi.org/10.3390/molecules200916852
Siddiqui N, Andalip Bawa S, Ali R, Afzal O, Akhtar MJ, Azad B, Kumar R (2011) Antidepressant potential of nitrogen-containing heterocyclic moieties: an updated review. J Pharm Bioallied Sci 3:194–212. https://doi.org/10.4103/0975-7406.80765
Sokolova AS, Yarovaya OI, Bormotov NI, Shishkina LN, Salakhutdinov NF (2018) Synthesis and antiviral activity of camphor-based 1,3-thiazolidin-4-one and thiazole derivatives as Orthopoxvirus-reproduction inhibitors. Med Chem Commun 9:1746–1753. https://doi.org/10.1039/C8MD00347E
Goel A, Agarwal N, Singh FV, Sharon A, Tiwari P, Dixit M, Pratap R, Srivastava AK, Maulik PR, Ram VJ (2004) Antihyperglycemic activity of 2-methyl-3, 4, 5-triaryl-1H-pyrroles in SLM and STZ models. Bioorg Med Chem Lett 14:1089–1092. https://doi.org/10.1016/j.bmcl.2004.01.009
Amir M, Javed SA, Kumar H (2007) Pyrimidine as antiinflammatory agent: a review. Indian J Pharm Sci 69(3):337–343. https://doi.org/10.4103/0250-474X.34540
Li W, Zhao SJ, Gao F, Lv ZS, Tu JY, Xu Z (2018) Synthesis and in vitro anti-tumor, anti-mycobacterial and anti-HIV activities of diethylene-glycol-tethered bis-isatin derivatives. Chem Select 3:10250–10254. https://doi.org/10.1002/slct.201802185
Khattab TA, Rehan MA (2018) A review on synthesis of nitrogen-containing heterocyclic dyes for textile fibers—part 2: fused heterocycles. Egypt J Chem 61:989–1018. https://doi.org/10.21608/ejchem.2018.4131.1363
Lamberth C, Dinges J (2012) Bioactive heterocyclic compound classes: agrochemicals. Wiley-VCH Verlag GmbH & Co, KGaA
Anastas PT, Warner JC (1998) Green chem theory and practice. Oxford Univ. Press, New York
Zhi S, Ma X, Zhang W (2019) Consecutive multicomponent reactions for the synthesis of complex molecules. Org Biomol Chem 17:7632–7650. https://doi.org/10.1039/C9OB00772E
Ibarra IA, Islas-Jácome A, González-Zamora E (2018) Synthesis of polyheterocycles via multicomponent reactions. Org Biomol Chem 16:1402–1418. https://doi.org/10.1039/C7OB02305G
Flores-Reyes JC, del Vanesa C, Cotlame-Salinas IA, Ibarra E-Z, Islas-Jácome A (2023) Pseudo-multicomponent reactions. RSC Adv 13:16091–16125. https://doi.org/10.1039/D3RA02746E
Graziano G, Stefanachi A, Contino M, Prieto-Díaz R, Ligresti A, Kumar P, Scilimati A, Sotelo E, Leonetti F (2023) Multicomponent reaction-assisted drug discovery: a time- and cost-effective green approach speeding up identification and optimization of anticancer drugs. Int J Mol Sci 24:6581. https://doi.org/10.3390/ijms24076581
Thirupathi D, Ramakanth P, Surjyakanta R, Sreekantha Babu J (2023) A concise review of multicomponent reactions using novel heterogeneous catalysts under microwave irradiation. Catalysts 13:1034. https://doi.org/10.3390/catal13071034
Kaddouri Y, Abrigach F, El Yousfi B, Hammouti B, El KM, Alsalme A, Al-Zaqri N, Warad I, Touzani R (2021) New heterocyclic compounds: synthesis, antioxidant activity and computational insights of nano-antioxidant as ascorbate peroxidase inhibitor by various cyclodextrins as drug delivery systems. Curr Drug Deliv 18:334–349. https://doi.org/10.2174/1567201817999201001205627
Nishiyama T, Hashiguchi Y, Sakata T, Sakaguchi T (2023) Antioxidant activity of the fused heterocyclic compounds, 1,2,3,4-tetrahydroquinolines, and related compounds—effect of ortho-substituents. Polym Degrad Stabil 79:225–230. https://doi.org/10.1016/S0141-3910(02)00285-9
Babizhayev MA, Deyev AI, Yermakovea VN, Brikman IV, Bours J (2004) Lipid peroxidation and cataracts: N-acetylcarnosine as a therapeutic tool to manage age-related cataracts in human and in canine eyes. Drugs in R & D 5:125–139. https://doi.org/10.2165/00126839-200405030-00001
Liu L, Meydani M (2002) Combined vitamin C and E supplementation retards early progression of arteriosclerosis in heart transplant patients. Nutr Rev 60:368–371. https://doi.org/10.1301/00296640260385810
Al-Messri ZAK (2023) Synthesis, characterization, and effectiveness of pyranopyrimidine derivatives as multi-function additive for lubricating oils. Chem Methodol 7(8):581–593. https://doi.org/10.22034/chemm.2023.393061.1669
Hossaini ZS, Zareyee D, Sheikholeslami-Farahani F, Vaseghi S, Zamani A (2017) ZnO-NR as the efficient catalyst for the synthesis of new thiazole and cyclopentadienone phosphonate derivatives in water. Heteroat Chem 28:e21362. https://doi.org/10.1002/hc.21362
Ezzatzadeh E, Farjam MH, Rustaiyan A (2012) Comparative evaluation of antioxidant and antimicrobial activity of crude extract and secondary metabolites isolated from Artemisia kulbadica. Asian Pac J Trop Disease 2:S431–S434. https://doi.org/10.1016/S2222-1808(12)60198-4
Yavari I, Hossaini ZS, Souri S, Seyfi S (2009) Diastereoselective synthesis of fused [1,3]thiazolo [1,3] oxazins and [1,3] oxazino [2,3-b][1,3] benzothiazoles. Mol Divers 13:439–443. https://doi.org/10.1007/s11030-009-9128-x
Baghernejad B, Sharifi Soltani N (2022) Cerium oxide/alumminium oxide-nanocatalyst promoted the production of dihydropyrano[c]chromene derivatives. Asian J Green Chem 6(2):166–174. https://doi.org/10.22034/ajgc.2022.2.6
Hoseini Z, Davoodnia A, Pordel M (2021) Another successful application of newly prepared GO-SiC3-NH3-H2PW as highly efficient nanocatalyst for fast synthesis of tetrahydrobenzo[b]pyrans. Adv J Chem A 4(1):68–77. https://doi.org/10.22034/ajca.2021.259593.1228
Mowlazadeh Haghighi S, Purkhosrow A, Khalafi-Nezhad A, Oftadehgan S (2022) One pot synthesis of heterocyclic dihydroquinoline analogs incorporating quinoline and pyrimidine fused rings in condensation reaction using NCTDSS as a catalyst. Asian J Green Chem 6(3):203222. https://doi.org/10.22034/ajgc.2022.3.3
Dianat M, Zare A, Hosainpour M (2021) Efficient protocol for the production of pyrimido[4,5-b] quinolines using an organic-inorganic hybrid catalyst. J Med Nanomater Chem 3(4):255–262. https://doi.org/10.48309/JMNC.2021.4.3
Rustaiyan A, Ezzatzadeh E (2011) Sesquiterpene lactones and penta methoxylated flavone from Artemisia kulbadica. Asian J Chem 23:1774–1776
Moosavi-Zare AR, Goudarziafshar H, Jalilian Z, Hosseinabadi F (2022) Efficient Pseudo-Six-Component Synthesis of Tetrahydro-pyrazolopyridines Using [Zn-2BSMP]Cl2. Chem Methodol 6(8):571–581. https://doi.org/10.22034/chemm.2022.334202.1456
Rajabi M, Hossaini ZS, Khalilzadeh MA, Datta Sh, Halder M, Mousa SA (2015) Synthesis of a new class of furo[3,2-C]coumarins and its anticancer activity. J Photochem Photobio B: Biol 148:66–72. https://doi.org/10.1016/j.jphotobiol.2015.03.027
Khazaei R, Khazaei A, Nasrollahzadeh M (2023) Horsetail assisted green synthesis of Fe3O4@SiO2-Pd: a reusable and highly efficient magnetically separable catalyst for Suzuki coupling reactions. J Appl Organomet Chem 3(2):123–133. https://doi.org/10.22034/jaoc.2023.397315.1079
SeifiMansour S, Ezzatzadeh E, Safarkar R (2019) In vitro evaluation of its antimicrobial effect of the synthesized Fe3O4 nanoparticles using Persea Americana extract as a green approach on two standard strains. Asian J Green Chem 3:353–365
Naderi S, Sandaroos R, Peiman S, Maleki B (2023) Synthesis and characterization of a novel crowned schiff base ligand linked to ionic liquid and application of its Mn(III) complex in the epoxidation of olefins. Chem Methodol 7(5):392–404. https://doi.org/10.22034/chemm.2023.385248.1651
Rostami-Charati F, Hossaini ZS, Rostamian R, Zamani A, Abdoli M (2017) Green synthesis of indol-2-one derivatives from N-alkylisatins in the presence of KF/clinoptilolite nanoparticles. Chem Heterocycl Comp 53:480–483. https://doi.org/10.1007/s10593-017-2077-x
Hakimi F, Golrasan E (2023) Zinc sulfide (ZnS) nanoparticles: an effective catalyst for synthesis of benzoxazole derivatives. Asian J Green Chem 7(1):47–53. https://doi.org/10.22034/ajgc.2023.1.6
Hakimi F, Taghvaee M, Golrasan E (2023) Synthesis of benzoxazole derivatives using Fe3O4@SiO2-SO3H nanoparticles as a useful and reusable heterogeneous catalyst without using a solvent. Adv J Chem A 6(2):188–197. https://doi.org/10.22034/ajca.2023.393949.1364
Rezayati S, Sheikholeslami-Farahani F, Hossaini ZS, Ha**asiri R, Afshari Sharif Abad S (2016) Regioselctive thiocyanation of aromatic and heteroaromatic compounds using a novel bronsted acidic ionic liquid. Comb Chem High Throughput Screen 9:720–727. https://doi.org/10.2174/1386207319666160709191851
Taghavi R, Rostamnia S (2022) Four-component synthesis of polyhydroquinolines via unsymmetrical hantzsch reaction employing Cu-IRMOF-3 as a robust heterogeneous catalyst. Chem Methodol 6(9):639–648. https://doi.org/10.22034/chemm.2022.340599.1511
Rostami Charati F, Hossaini ZS, Hosseini-Tabatabaei MR (2011) A simple synthesis of oxaphospholes. Phosphorus Sulfur Silicon Relat Elem 186:1443–1448. https://doi.org/10.1080/10426507.2010.515953
Baghernejad B, Hojjatitaromsari SM (2022) Preparation of polyhydroquinolines using nano cerium (IV) oxide/zinc oxide as a catalyst. J Appl Organomet Chem 2(2):74–80. https://doi.org/10.22034/jaoc.2022.340645.1054
Sajjadi-Ghotbabadi H, Javanshir SH, Rostami-Charati F (2016) Nano KF/clinoptilolite: an effective heterogeneous base nanocatalyst for synthesis of substituted quinolines in water. Catal Lett 146:338–344. https://doi.org/10.1007/s10562-015-1652-y
Farajpour M, Vahdat SM, Baghbanian SM, Hatami M (2023) Ag-SiO2 nanoparticles: benign, expedient, and facile nano catalyst in synthesis of decahydroacridines. Chem Methodol 7(7):540–551. https://doi.org/10.22034/chemm.2023.386678.1653
Hakimi F, Mousavian B, Banifatemeh F, Golrasan E (2021) ZrCl4@Arabic gum: an effective and environmentally friendly catalyst for the preparation of 14-aryl-14H-dibenzo[a, j]xanthene derivatives at ambient temperature without solvent. Asian J Green Chem 5(4):378–386. https://doi.org/10.22034/ajgc.2021.304712.1314
Mhaibes RM, Arzehgar Z, Mirzaei Heydari M, Fatolahi L (2023) ZnO nanoparticles: a highly efficient and recyclable catalyst for tandem Knoevenagel–Michael–Cyclocondensation reaction. Asian J Green Chem 7(1):1–8. https://doi.org/10.22034/ajgc.2023.1.1
Yavari I, Nematpour M, Hossaini ZS (2010) Ph3P-mediated one-pot synthesis of functionalized 3, 4-dihydro-2H-1, 3-thiazines from N, N′-dialkylthioureas and activated acetylenes in water. Monatsh fur Chem 141:229–232. https://doi.org/10.1007/s00706-009-0247-y
Hamidi Zare S, Ghorayshi Nejad MS, Saberi A, Rostami E (2022) A highly efficient and sustainable synthesis of 1-[(1,3-thiazol-2-ylamino) methyl]-2-naphthols under solvent-free conditions using graphene oxide substituted ethane sulfonic acid catalyst. J Med Nanomater Chem 4(3):211–224. https://doi.org/10.48309/jmnc.2022.3.4
Yavari I, Sabbaghan M, Hossaini ZS (2008) Proline-promoted efficient synthesis of 4-aryl-3,4-dihydro-2h,5h-pyrano[3,2-c]chromene-2,5-diones in aqueous media. Synlett 8:1153–1154. https://doi.org/10.1055/s-2008-1072656
Abed Nashaan F, Sameer Al-Rawi M (2023) Design, synthesis, and biological activity of new thiazolidine-4-one derived from symmetrical 4-amino-1,2,4-triazole. Chem Methodol 7(2):106–111. https://doi.org/10.22034/chemm.2023.362512.1610
Ezzatzadeh E, Hossaini ZS, Rostamian R, Vaseghi S, Mousavi SF (2017) Fe3O4 magnetic nanoparticles (MNPs) as reusable catalyst for the synthesis of chromene derivatives using multicomponent reaction of 4-hydroxycumarin basis on cheletropic reaction. J Heterocycl Chem 54(5):2906–2911. https://doi.org/10.1002/jhet.2900
Hani YZ, Zainal IG, Asker FW, Jasim LH (2023) Study of Some Heterocyclic Compounds Made from a New 4(3H)-Quinazolinone and Their Biological and Antioxidant Activities. Chem Methodol 7(5):372–382. https://doi.org/10.22034/chemm.2023.379917.1641
Ezzatzadeh E, Sheikholeslami-Farahani F, Yadollahzadeh K, Rezayati S (2021) Highly Efficient Reusable Carboxy Group Functionalized Imidazolium Salts for a Simple and Cost-effective Preparation of pyrano[2,3-d]pyrimidinone Derivatives. Comb Chem High Throughput Screen 24:1465–1475. https://doi.org/10.2174/1386207323666201007154343
Farjam MH, Rustaiyan A, Ezzatzadeh E, Jassbi AR (2013) Labdane- type diterpene and two flavones from Salvia sharifii Rech. f. & Esfan. and their biological activities. Iran J Pharm Res 12(2):395–399. https://doi.org/10.22037/ijpr.2013.1297
Rezayati S, Kalantari F, Ramazani A, Ezzatzadeh E (2021) Isoquinolinium-N-sulfonic acid thiocyanate/H2O2 as an efficient reagent for the thiocyanation of N-bearing. J Sulfur Chem 42(5):575–590. https://doi.org/10.1080/17415993.2021.1929230
Ghafuri H, Zargari M, Emami A (2023) Green and eco-friendly synthesis of 2-amino-3-cyano-4h-chromene derivatives via eggshell/Fe3O4 as a biodegradable polymer matrix nanocomposite. Asian J Green Chem 7(1):54–69. https://doi.org/10.22034/ajgc.2023.1.7
Baghernejad B, Zakariayi A (2022) One-Pot synthesis of oxindoles derivatives as effective antimicrobial agents by nano-magnesium aluminate as an effective aatalyst. J Med Nanomater Chem 4(3):225–233. https://doi.org/10.48309/jmnc.2022.3.5
Hamedani NF, Zamani Hargalani F, Rostami-Charati F (2021) Biosynthesis of Cu/KF/Clinoptilolite@MWCNTs nanocomposite and its application as a recyclable nanocatalyst for the synthesis of new Schiff base of benzoxazine derivatives and reduction of organic pollutants. Mol Divers 26(4):2069–2083. https://doi.org/10.1007/s11030-021-10316-1
Dey S, Basak P, Sarkar S, Ghosh P (2022) A design for convenient and greener root towards one-pot multi-component synthesis of substituted pyrano-dichromeneo-dione and chromeno-pyrido-pyrimidinone derivatives using rice husk based heterogeneous catalyst. Asian J Green Chem 6(1):24–39. https://doi.org/10.22034/ajgc.2022.1.3
Fattahi M, Ezzatzadeh E, Jalilian R, Taheri A (2021) Micro solid phase extraction of cadmium and lead on a new ion-imprinted hierarchical mesoporous polymer via dual-template method in river water and fish muscles: optimization by experimental design. J Hazard Mater 403:123716. https://doi.org/10.1016/j.jhazmat.2020.123716
Rustaiyan A, Masoudi S, Ezzatzadeh E, Akhlaghi H, Aboli J (2011) Composition of the aerial part, flower, leaf and stem oils of eremostachysmacrophyllamontbr. & Auch. and Eremostachyslabiosa bunge. from Iran. J Essent Oil Bear Pl 14:84–88. https://doi.org/10.1080/0972060X.2011.10643904
Hakimi F, Sharifi-Zarchi A, Golrasan E (2023) Bifunctional polyethylene glycol/ethylenediamine nanomagnetic phase-transfer catalyst: preparation, characterization, and application in knoevenagel condensation. Chem Methodol 7(6):489–498. https://doi.org/10.22034/chemm.2023.392041.1667
Mohammed AY, Ahamed LS (2022) Synthesis and characterization of new substituted Coumarin derivatives and study their biological activity. Chem Methodol 6(11):813–822. https://doi.org/10.22034/CHEMM.2022.349124.1569
Meresht AS, Ezzatzadeh E, Dehbandi B, Salimifard M, Rostamian R (2022) Fe3O4/CuO nanocomposite promoted green synthesis of functionalized quinazolines using water extract of lettuce leaves as green media: study of antioxidant activity. Polycycl Aromat Compd 42(7):4793–4808. https://doi.org/10.1080/10406638.2021.1913426
Muhiebes RM, Fatolahi L, Sajjadifar S (2023) L-proline catalyzed multicomponent reaction for simple and efficient synthesis of tetrahydropyridines derivatives. Asian J Green Chem 7(2):121–131. https://doi.org/10.22034/ajgc.2023.393683.1380
Jalilian R, Ezzatzadeh E, Taheri A (2021) A novel self-assembled gold nanoparticles-molecularly imprinted modified carbon ionic liquid electrode with high sensitivity and selectivity for the rapid determination of bisphenol A leached from plastic containers. J Environ Chem Eng 9:105513. https://doi.org/10.1016/j.jece.2021.105513
Karim Hamad B, Ahamed MR (2022) Synthesis and characterization of heterocyclic compounds derived from 2-mercaptobenzothiozole and study of their biological activities. Chem Methodol 6(5):409–417. https://doi.org/10.22034/chemm.2022.332101.1447
Narayan S, Muldoon J, Finn MG, Fokin VV, Kolb HC, Sharpless KB (2005) On water”: unique reactivity of organic compounds in aqueous suspension. Angew Chem Int Ed 44(21):3157–3159. https://doi.org/10.1002/anie.200462883
Chanda A, Fokin VV (2009) Organic synthesis “on water.” Chem Rev 109(2):725–748. https://doi.org/10.1021/cr800448q
Butler RN, Coyne AG (2016) Organic synthesis reactions on-water at the organic–liquid water interface. Org Biomol Chem 14:9945–9960. https://doi.org/10.1039/C6OB01724J
Min W, Wang T, Li W, Zhang Q, Zhang B, Chen K, Peng S, Li G, Huang J, Wang Q, Wang C (2023) Water-favored reaction mechanism for selective catalytic conversion of 2-methylfuran to 1,4-pentanediol. Chem Eng J 461:141944. https://doi.org/10.1016/j.cej.2023.141944
Saundane AR, Nandibeoor MK (2015) Synthesis, characterization, and biological evaluation of Schiff bases containing indole moiety and their derivatives. Monatsh Chem 146:1751–1761. https://doi.org/10.1007/s00706-015-1440-9
Bidchol AM, Wilfred A, Abhijna P, Harish R (2011) Free radical scavenging activity of aqueous and ethanolic extract of Brassica oleracea L. var. italica. Food Bioprocess Tech 4:1137–1143. https://doi.org/10.1007/s11947-009-0196-9
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948. https://doi.org/10.1021/jf00018a005
Yen GC, Duh PD (1994) Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem 42:629–632. https://doi.org/10.1021/jf00039a005
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 49(8):4083–4089. https://doi.org/10.1021/jf0103572
Acknowledgements
The author sincerely would like to acknowledge the Islamic Azad University of Ardabil for its support.
Author information
Authors and Affiliations
Contributions
All of Authors contributed in all of section of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest are disclosed by the writers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasani, L., Ezzatzadeh, E. & Hossaini, Z. Green synthesis and investigation of antioxidant and antibacterial activity of new derivatives of chromenoazepines employing CuO/TiO2@MWCNTs. Mol Divers (2024). https://doi.org/10.1007/s11030-023-10803-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10803-7