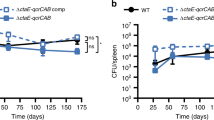
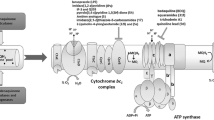
Abstract
The terminal oxidases of the oxidative phosphorylation pathway play a significant role in the survival and growth of M. tuberculosis, targeting these components lead to inhibition of M. tuberculosis. Many drug candidates targeting various components of the electron transport chain in M. tuberculosis have recently been discovered. The cytochrome bc1-aa3 supercomplex is one of the most important components of the electron transport chain in M. tuberculosis, and it has emerged as the novel target for several promising candidates. There are two cryo-electron microscopy structures (PDB IDs: 6ADQ and 6HWH) of the cytochrome bc1-aa3 supercomplex that aid in the development of effective and potent inhibitors for M. tuberculosis. In recent years, a number of potential candidates targeting the QcrB subunit of the cytochrome bc1 complex have been developed. In this review, we describe the recently identified inhibitors that target the electron transport chain's terminal oxidase enzyme in M. tuberculosis, specifically the QcrB subunit of the cytochrome bc1 complex.




Similar content being viewed by others
References
Smith I (2003) Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 16(3):463–496. https://doi.org/10.1128/CMR.16.3.463-496.2003
Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, Jensen P, Bayona J (2010) Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375(9728):1830–1843. https://doi.org/10.1016/S0140-6736(10)60410-2
Pawlowski A, Jansson M, Skold M, Rottenberg ME, Kallenius G (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8(2):e1002464. https://doi.org/10.1371/journal.ppat.1002464
Fogel N (2015) Tuberculosis: a disease without boundaries. Tuberculosis (Edinb) 95(5):527–531. https://doi.org/10.1016/j.tube.2015.05.017
Chan ED, Iseman MD (2002) Current medical treatment for tuberculosis. BMJ 325(7375):1282–1286. https://doi.org/10.1136/bmj.325.7375.1282
Ray S, Talukdar A, Kundu S, Khanra D, Sonthalia N (2013) Diagnosis and management of miliary tuberculosis: current state and future perspectives. Ther Clin Risk Manag 9:9–26. https://doi.org/10.2147/TCRM.S29179
Zajaczkowski T (2012) Genitourinary tuberculosis: historical and basic science review: past and present. Cent European J Urol 65(4):182–187. https://doi.org/10.5173/ceju.2012.04.art1
WHO (2020) Global Tuberculosis Report. 2020.
Organization WH (2016) What is DOTS? A guide to understanding the WHO recommended TB control strategy known as DOTS. 1999. WHO/CDS/CPC/TB/99.270,
Nunn P, Porter J, Winstanley P (1993) Thiacetazone—avoid like poison or use with care? Trans R Soc Trop Med Hyg 87(5):578–582. https://doi.org/10.1016/0035-9203(93)90096-9
Roy KK, Wani MA (2020) Emerging opportunities of exploiting mycobacterial electron transport chain pathway for drug-resistant tuberculosis drug discovery. Expert Opin Drug Discov 15(2):231–241. https://doi.org/10.1080/17460441.2020.1696771
Machado D, Girardini M, Viveiros M, Pieroni M (2018) Challenging the drug-likeness dogma for new drug discovery in tuberculosis. Front Microbiol 9:1367. https://doi.org/10.3389/fmicb.2018.01367
Foo CS-Y, Pethe K, Lupien A (2020) Oxidative phosphorylation—an update on a new, essential target space for drug discovery in Mycobacterium tuberculosis. Appl Sci 10(7):2339. https://doi.org/10.3390/app10072339
Advani MJ, Siddiqui I, Sharma P, Reddy H (2012) Activity of trifluoperazine against replicating, non-replicating and drug resistant M. tuberculosis. PLoS ONE 7(8):e44245. https://doi.org/10.1371/journal.pone.0044245
Yano T, Li LS, Weinstein E, Teh JS, Rubin H (2006) Steady-state kinetics and inhibitory action of antitubercular phenothiazines on Mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2). J Biol Chem 281(17):11456–11463. https://doi.org/10.1074/jbc.M508844200
Lechartier B, Cole ST (2015) Mode of Action of Clofazimine and Combination Therapy with Benzothiazinones against Mycobacterium tuberculosis. Antimicrob Agents Chemother 59(8):4457–4463. https://doi.org/10.1128/AAC.00395-15
Mirnejad R, Asadi A, Khoshnood S, Mirzaei H, Heidary M, Fattorini L, Ghodousi A, Darban-Sarokhalil D (2018) Clofazimine: a useful antibiotic for drug-resistant tuberculosis. Biomed Pharmacother 105:1353–1359. https://doi.org/10.1016/j.biopha.2018.06.023
Lu Y, Zheng M, Wang B, Fu L, Zhao W, Li P, Xu J, Zhu H, ** H, Yin D, Huang H, Upton AM, Ma Z (2011) Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob Agents Chemother 55(11):5185–5193. https://doi.org/10.1128/AAC.00699-11
Xu J, Lu Y, Fu L, Zhu H, Wang B, Mdluli K, Upton AM, ** H, Zheng M, Zhao W, Li P (2012) In vitro and in vivo activity of clofazimine against Mycobacterium tuberculosis persisters. Int J Tuberc Lung Dis 16(8):1119–1125. https://doi.org/10.5588/ijtld.11.0752
Barry VC, Conalty ML (1958) Antituberculosis activity in the phenazine series. II. N3-substituted anilinoaposafranines (rimino-compounds) and some derivatives. Am Rev Tuberc 78(1):62–73. https://doi.org/10.1164/artpd.1958.78.1.62
Shetye GS, Franzblau SG, Cho S (2020) New tuberculosis drug targets, their inhibitors, and potential therapeutic impact. Transl Res 220:68–97. https://doi.org/10.1016/j.trsl.2020.03.007
Sotgiu G, Tiberi S, Centis R, D’Ambrosio L, Fuentes Z, Zumla A, Migliori GB (2017) Applicability of the shorter “Bangladesh regimen” in high multidrug-resistant tuberculosis settings. Int J Infect Dis 56:190–193. https://doi.org/10.1016/j.ijid.2016.10.021
Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL (2010) Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182(5):684–692. https://doi.org/10.1164/rccm.201001-0077OC
Harbut MB, Yang B, Liu R, Yano T, Vilcheze C, Cheng B, Lockner J, Guo H, Yu C, Franzblau SG, Petrassi HM, Jacobs WR Jr, Rubin H, Chatterjee AK, Wang F (2018) Small molecules targeting Mycobacterium tuberculosis type II NADH dehydrogenase exhibit antimycobacterial activity. Angew Chem Int Ed Engl 57(13):3478–3482. https://doi.org/10.1002/anie.201800260
Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R, Ristic Z, Lill H, Dorange I, Guillemont J, Bald D, Andries K (2007) Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol 3(6):323–324. https://doi.org/10.1038/nchembio884
Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307(5707):223–227. https://doi.org/10.1126/science.1106753
Biukovic G, Basak S, Manimekalai MS, Rishikesan S, Roessle M, Dick T, Rao SP, Hunke C, Gruber G (2013) Variations of subunit varepsilon of the Mycobacterium tuberculosis F1Fo ATP synthase and a novel model for mechanism of action of the tuberculosis drug TMC207. Antimicrob Agents Chemother 57(1):168–176. https://doi.org/10.1128/AAC.01039-12
Kundu S, Biukovic G, Gruber G, Dick T (2016) Bedaquiline targets the epsilon subunit of mycobacterial F-ATP synthase. Antimicrob Agents Chemother 60(11):6977–6979. https://doi.org/10.1128/AAC.01291-16
Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, Mc Neeley DF (2009) The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 360(23):2397–2405. https://doi.org/10.1056/NEJMoa0808427
Matteelli A, Carvalho AC, Dooley KE, Kritski A (2010) TMC207: the first compound of a new class of potent anti-tuberculosis drugs. Future Microbiol 5(6):849–858. https://doi.org/10.2217/fmb.10.50
Fox GJ, Menzies D (2013) A review of the evidence for using bedaquiline (TMC207) to treat multi-drug resistant tuberculosis. Infect Dis Ther 2(2):123–144. https://doi.org/10.1007/s40121-013-0009-3
Pontali E, Sotgiu G, Tiberi S, D’Ambrosio L, Centis R, Migliori GB (2017) Cardiac safety of bedaquiline: a systematic and critical analysis of the evidence. Eur Respir J. https://doi.org/10.1183/13993003.01462-2017
Guglielmetti L, Tiberi S, Burman M, Kunst H, Wejse C, Togonidze T, Bothamley G, Lange C, Tbnet, of the TQs (2018) QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: a Tuberculosis Network European Trialsgroup (TBnet) study. Eur Respir J https://doi.org/10.1183/13993003.00537-2018
Choi PJ, Sutherland HS, Tong AST, Blaser A, Franzblau SG, Cooper CB, Lotlikar MU, Upton AM, Guillemont J, Motte M, Queguiner L, Andries K, Van den Broeck W, Denny WA, Palmer BD (2017) Synthesis and evaluation of analogues of the tuberculosis drug bedaquiline containing heterocyclic B-ring units. Bioorg Med Chem Lett 27(23):5190–5196. https://doi.org/10.1016/j.bmcl.2017.10.042
Patel H, Pawara R, Pawara K, Ahmed F, Shirkhedkar A, Surana S (2019) A structural insight of bedaquiline for the cardiotoxicity and hepatotoxicity. Tuberculosis (Edinb) 117:79–84. https://doi.org/10.1016/j.tube.2019.06.005
van Heeswijk RP, Dannemann B, Hoetelmans RM (2014) Bedaquiline: a review of human pharmacokinetics and drug-drug interactions. J Antimicrob Chemother 69(9):2310–2318. https://doi.org/10.1093/jac/dku171
Xu J, Converse PJ, Upton AM, Mdluli K, Fotouhi N, Nuermberger EL (2021) Comparative efficacy of the novel diarylquinoline TBAJ-587 and bedaquiline against a resistant Rv0678 mutant in a mouse model of tuberculosis. Antimicrob Agents Chemother 65(4):e02418-02420. https://doi.org/10.1128/AAC.02418-20
Sarathy JP, Ragunathan P, Shin J, Cooper CB, Upton AM, Gruber G, Dick T (2019) TBAJ-876 retains Bedaquiline’s activity against subunits c and epsilon of Mycobacterium tuberculosis F-ATP synthase. Antimicrob Agents Chemother 63(10):e01191-e11119. https://doi.org/10.1128/AAC.01191-19
Sutherland HS, Tong AST, Choi PJ, Blaser A, Conole D, Franzblau SG, Lotlikar MU, Cooper CB, Upton AM, Denny WA, Palmer BD (2019) 3,5-Dialkoxypyridine analogues of bedaquiline are potent antituberculosis agents with minimal inhibition of the hERG channel. Bioorg Med Chem 27(7):1292–1307. https://doi.org/10.1016/j.bmc.2019.02.026
Hotra A, Ragunathan P, Ng PS, Seankongsuk P, Harikishore A, Sarathy JP, Saw WG, Lakshmanan U, Sae-Lao P, Kalia NP, Shin J, Kalyanasundaram R, Anbarasu S, Parthasarathy K, Pradeep CN, Makhija H, Droge P, Poulsen A, Tan JHL, Pethe K, Dick T, Bates RW, Gruber G (2020) Discovery of a novel mycobacterial F-ATP synthase inhibitor and its potency in combination with diarylquinolines. Angew Chem Int Ed Engl 59(32):13295–13304. https://doi.org/10.1002/anie.202002546
Dhulap A, Banerjee P (2021) ATP synthase, an emerging target in TB drug discovery: review of SAR and clinical pharmacology of diarylquinoline inhibitors. Curr Drug Targets 22(11):1207–1221. https://doi.org/10.2174/1389450122666210122084332
Appetecchia F, Consalvi S, Scarpecci C, Biava M, Poce G (2020) SAR analysis of small molecules interfering with energy-metabolism in Mycobacterium tuberculosis. Pharmaceuticals (Basel) 13(9):227. https://doi.org/10.3390/ph13090227
Preiss L, Langer JD, Yildiz O, Eckhardt-Strelau L, Guillemont JE, Koul A, Meier T (2015) Structure of the mycobacterial ATP synthase Fo rotor ring in complex with the anti-TB drug bedaquiline. Sci Adv 1(4):e1500106. https://doi.org/10.1126/sciadv.1500106
Lu P, Heineke MH, Koul A, Andries K, Cook GM, Lill H, van Spanning R, Bald D (2015) The cytochrome bd-type quinol oxidase is important for survival of Mycobacterium smegmatis under peroxide and antibiotic-induced stress. Sci Rep 5(1):10333. https://doi.org/10.1038/srep10333
Mascolo L, Bald D (2020) Cytochrome bd in Mycobacterium tuberculosis: A respiratory chain protein involved in the defense against antibacterials. Prog Biophys Mol Biol 152:55–63. https://doi.org/10.1016/j.pbiomolbio.2019.11.002
Jünemann S, Wrigglesworth JM, Rich PR (1997) Effects of decyl-aurachin D and reversed electron transfer in cytochrome bd. Biochemistry 36(31):9323–9331. https://doi.org/10.1021/bi970055m
Moraski GC, Markley LD, Hipskind PA, Boshoff H, Cho S, Franzblau SG, Miller MJ (2011) Advent of Imidazo[1,2-a]pyridine-3-carboxamides with potent multi- and extended drug resistant antituberculosis activity. ACS Med Chem Lett 2(6):466–470. https://doi.org/10.1021/ml200036r
Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim SA, Nam J, Kang H, Kwon H, Oh CT, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SP, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han SJ, No Z, Lee J, Brodin P, Cho SN, Nam K, Kim J (2013) Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19(9):1157–1160. https://doi.org/10.1038/nm.3262
Gong H, Li J, Xu A, Tang Y, Ji W, Gao R, Wang S, Yu L, Tian C, Li J, Yen HY, Man Lam S, Shui G, Yang X, Sun Y, Li X, Jia M, Yang C, Jiang B, Lou Z, Robinson CV, Wong LL, Guddat LW, Sun F, Wang Q, Rao Z (2018) An electron transfer path connects subunits of a mycobacterial respiratory supercomplex. Science 362(6418):eaat8923. https://doi.org/10.1126/science.aat8923
Kashket ER (1985) The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol 39(1):219–242. https://doi.org/10.1146/annurev.mi.39.100185.001251
Cook GM, Berney M, Gebhard S, Heinemann M, Cox RA, Danilchanka O, Niederweis M (2009) Physiology of mycobacteria. Adv Microb Physiol 55(81–182):318–319. https://doi.org/10.1016/S0065-2911(09)05502-7
Cook GM, Hards K, Vilcheze C, Hartman T, Berney M (2014) Energetics of respiration and oxidative phosphorylation in mycobacteria. Microbiol Spectr 2(3):389–409. https://doi.org/10.1128/microbiolspec.MGM2-0015-2013
Vilcheze C, Weinrick B, Leung LW, Jacobs WR Jr (2018) Plasticity of Mycobacterium tuberculosis NADH dehydrogenases and their role in virulence. Proc Natl Acad Sci U S A 115(7):1599–1604. https://doi.org/10.1073/pnas.1721545115
Iqbal IK, Bajeli S, Akela AK, Kumar A (2018) Bioenergetics of mycobacterium: an emerging landscape for drug discovery. Pathogens 7(1):1–24. https://doi.org/10.3390/pathogens7010024
Pecsi I, Hards K, Ekanayaka N, Berney M, Hartman T, Jacobs WR Jr, Cook GM (2014) Essentiality of succinate dehydrogenase in Mycobacterium smegmatis and its role in the generation of the membrane potential under hypoxia. MBio 5(4):e01093-01014. https://doi.org/10.1128/mBio.01093-14
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393(6685):537–544. https://doi.org/10.1038/31159
Gao X, **n Y, Bell PD, Wen J, Blankenship RE (2010) Structural analysis of alternative complex III in the photosynthetic electron transfer chain of Chloroflexus aurantiacus. Biochemistry 49(31):6670–6679. https://doi.org/10.1021/bi100858k
Pereira MM, Refojo PN, Hreggvidsson GO, Hjorleifsdottir S, Teixeira M (2007) The alternative complex III from Rhodothermus marinus—a prototype of a new family of quinol:electron acceptor oxidoreductases. FEBS Lett 581(25):4831–4835. https://doi.org/10.1016/j.febslet.2007.09.008
Sun C, Benlekbir S, Venkatakrishnan P, Wang Y, Hong S, Hosler J, Tajkhorshid E, Rubinstein JL, Gennis RB (2018) Structure of the alternative complex III in a supercomplex with cytochrome oxidase. Nature 557(7703):123–126. https://doi.org/10.1038/s41586-018-0061-y
Wiseman B, Nitharwal RG, Fedotovskaya O, Schafer J, Guo H, Kuang Q, Benlekbir S, Sjostrand D, Adelroth P, Rubinstein JL, Brzezinski P, Hogbom M (2018) Structure of a functional obligate complex III2IV2 respiratory supercomplex from Mycobacterium smegmatis. Nat Struct Mol Biol 25(12):1128–1136. https://doi.org/10.1038/s41594-018-0160-3
Megehee JA, Hosler JP, Lundrigan MD (2006) Evidence for a cytochrome bcc-aa3 interaction in the respiratory chain of Mycobacterium smegmatis. Microbiology (Reading) 152(Pt 3):823–829. https://doi.org/10.1099/mic.0.28723-0
Sone N, Nagata K, Kojima H, Tajima J, Kodera Y, Kanamaru T, Noguchi S, Sakamoto J (2001) A novel hydrophobic diheme c-type cytochrome. Purification from Corynebacterium glutamicum and analysis of the QcrCBA operon encoding three subunit proteins of a putative cytochrome reductase complex. Biochim Biophys Acta 1503(3):279–290. https://doi.org/10.1016/s0005-2728(00)00205-x
Hunte C, Koepke J, Lange C, Roßmanith T, Michel H (2000) Structure at 2.3 Å resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure 8(6):669–684. https://doi.org/10.1016/s0969-2126(00)00152-0
Sousa FL, Alves RJ, Ribeiro MA, Pereira-Leal JB, Teixeira M, Pereira MM (2012) The superfamily of heme-copper oxygen reductases: types and evolutionary considerations. Biochim Biophys Acta 1817(4):629–637. https://doi.org/10.1016/j.bbabio.2011.09.020
Beites T, O’Brien K, Tiwari D, Engelhart CA, Walters S, Andrews J, Yang HJ, Sutphen ML, Weiner DM, Dayao EK, Zimmerman M, Prideaux B, Desai PV, Masquelin T, Via LE, Dartois V, Boshoff HI, Barry CE 3rd, Ehrt S, Schnap**er D (2019) Plasticity of the Mycobacterium tuberculosis respiratory chain and its impact on tuberculosis drug development. Nat Commun 10(1):4970. https://doi.org/10.1038/s41467-019-12956-2
Kalia NP, Hasenoehrl EJ, Ab Rahman NB, Koh VH, Ang MLT, Sajorda DR, Hards K, Gruber G, Alonso S, Cook GM, Berney M, Pethe K (2017) Exploiting the synthetic lethality between terminal respiratory oxidases to kill Mycobacterium tuberculosis and clear host infection. Proc Natl Acad Sci U S A 114(28):7426–7431. https://doi.org/10.1073/pnas.1706139114
Boshoff HI, Barry CE 3rd (2005) Tuberculosis - metabolism and respiration in the absence of growth. Nat Rev Microbiol 3(1):70–80. https://doi.org/10.1038/nrmicro1065
Chandrasekera NS, Berube BJ, Shetye G, Chettiar S, O’Malley T, Manning A, Flint L, Awasthi D, Ioerger TR, Sacchettini J, Masquelin T, Hipskind PA, Odingo J, Parish T (2017) Improved phenoxyalkylbenzimidazoles with activity against Mycobacterium tuberculosis appear to target QcrB. ACS Infect Dis 3(12):898–916. https://doi.org/10.1021/acsinfecdis.7b00112
Schnap**er D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK (2003) Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J Exp Med 198(5):693–704. https://doi.org/10.1084/jem.20030846
Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML (2005) Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A 102(43):15629–15634. https://doi.org/10.1073/pnas.0507850102
Berney M, Cook GM (2010) Unique flexibility in energy metabolism allows mycobacteria to combat starvation and hypoxia. PLoS ONE 5(1):e8614. https://doi.org/10.1371/journal.pone.0008614
Thierbach G, Kunze B, Reichenbach H, Höfle G (1984) The mode of action of stigmatellin, a new inhibitor of the cytochrome b-c1 segment of the respiratory chain. Biochimica. et Biophysica. Acta (BBA) - Bioenergetics 765(2):227–235. https://doi.org/10.1016/0005-2728(84)90017-3
Oettmeier W, Godde D, Kunze B, Höfle G (1985) Stigmatellin. A dual type inhibitor of photosynthetic electron transport. Biochimica et Biophysica Acta (BBA) - Bioenergetics 807(2):216–219. https://doi.org/10.1016/0005-2728(85)90125-2
Abrahams KA, Cox JA, Spivey VL, Loman NJ, Pallen MJ, Constantinidou C, Fernandez R, Alemparte C, Remuinan MJ, Barros D, Ballell L, Besra GS (2012) Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB. PLoS ONE 7(12):e52951. https://doi.org/10.1371/journal.pone.0052951
Cheng Y, Moraski GC, Cramer J, Miller MJ, Schorey JS (2014) Bactericidal activity of an imidazo[1, 2-a]pyridine using a mouse M. tuberculosis infection model. PLoS ONE 9(1):e87483. https://doi.org/10.1371/journal.pone.0087483
Bardhan KD, Hawkey CJ, Long RG, Morgan AG, Wormsley KG, Moules IK, Brocklebank D (1995) Lansoprazole versus ranitidine for the treatment of reflux oesophagitis. UK Lansoprazole Clinical Research Group. Aliment Pharmacol Ther 9(2):145–151. https://doi.org/10.1111/j.1365-2036.1995.tb00363.x
Rybniker J, Vocat A, Sala C, Busso P, Pojer F, Benjak A, Cole ST (2015) Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nat Commun 6(1):7659. https://doi.org/10.1038/ncomms8659
Moraski GC, Miller PA, Bailey MA, Ollinger J, Parish T, Boshoff HI, Cho S, Anderson JR, Mulugeta S, Franzblau SG, Miller MJ (2015) Putting Tuberculosis (TB) To Rest: Transformation of the Sleep Aid, Ambien, and “Anagrams” Generated Potent Antituberculosis Agents. ACS Infect Dis 1(2):85–90. https://doi.org/10.1021/id500008t
van der Westhuyzen R, Winks S, Wilson CR, Boyle GA, Gessner RK, Soares de Melo C, Taylor D, de Kock C, Njoroge M, Brunschwig C, Lawrence N, Rao SP, Sirgel F, van Helden P, Seldon R, Moosa A, Warner DF, Arista L, Manjunatha UH, Smith PW, Street LJ, Chibale K (2015) Pyrrolo[3,4-c]pyridine-1,3(2H)-diones: a novel antimycobacterial class targeting mycobacterial respiration. J Med Chem 58(23):9371–9381. https://doi.org/10.1021/acs.jmedchem.5b01542
Tang J, Wang B, Wu T, Wan J, Tu Z, Njire M, Wan B, Franzblauc SG, Zhang T, Lu X, Ding K (2015) Design, Synthesis, and Biological Evaluation of Pyrazolo[1,5-a]pyridine-3-carboxamides as Novel Antitubercular Agents. ACS Med Chem Lett 6(7):814–818. https://doi.org/10.1021/acsmedchemlett.5b00176
Moraski GC, Seeger N, Miller PA, Oliver AG, Boshoff HI, Cho S, Mulugeta S, Anderson JR, Franzblau SG, Miller MJ (2016) Arrival of Imidazo[2,1-b]thiazole-5-carboxamides: Potent Anti-tuberculosis Agents That Target QcrB. ACS Infect Dis 2(6):393–398. https://doi.org/10.1021/acsinfecdis.5b00154
Moraski GC, Deboosere N, Marshall KL, Weaver HA, Vandeputte A, Hastings C, Woolhiser L, Lenaerts AJ, Brodin P, Miller MJ (2020) Intracellular and in vivo evaluation of imidazo[2,1-b]thiazole-5-carboxamide anti-tuberculosis compounds. PLoS ONE 15(1):e0227224. https://doi.org/10.1371/journal.pone.0227224
Pissinate K, Villela AD, Rodrigues-Junior V, Giacobbo BC, Grams ES, Abbadi BL, Trindade RV, Roesler Nery L, Bonan CD, Back DF, Campos MM, Basso LA, Santos DS, Machado P (2016) 2-(Quinolin-4-yloxy)acetamides Are Active against Drug-Susceptible and Drug-Resistant Mycobacterium tuberculosis Strains. ACS Med Chem Lett 7(3):235–239. https://doi.org/10.1021/acsmedchemlett.5b00324
Phummarin N, Boshoff HI, Tsang PS, Dalton J, Wiles S, Barry Rd CE, Copp BR (2016) SAR and identification of 2-(quinolin-4-yloxy)acetamides as Mycobacterium tuberculosis cytochrome bc1 inhibitors. Medchemcomm 7(11):2122–2127. https://doi.org/10.1039/c6md00236f
Chandrasekera NS, Alling T, Bailey MA, Files M, Early JV, Ollinger J, Ovechkina Y, Masquelin T, Desai PV, Cramer JW, Hipskind PA, Odingo JO, Parish T (2015) Identification of Phenoxyalkylbenzimidazoles with Antitubercular Activity. J Med Chem 58(18):7273–7285. https://doi.org/10.1021/acs.jmedchem.5b00546
Tantry SJ, Markad SD, Shinde V, Bhat J, Balakrishnan G, Gupta AK, Ambady A, Raichurkar A, Kedari C, Sharma S, Mudugal NV, Narayan A, Naveen Kumar CN, Nanduri R, Bharath S, Reddy J, Panduga V, Prabhakar KR, Kandaswamy K, Saralaya R, Kaur P, Dinesh N, Guptha S, Rich K, Murray D, Plant H, Preston M, Ashton H, Plant D, Walsh J, Alcock P, Naylor K, Collier M, Whiteaker J, McLaughlin RE, Mallya M, Panda M, Rudrapatna S, Ramachandran V, Shandil R, Sambandamurthy VK, Mdluli K, Cooper CB, Rubin H, Yano T, Iyer P, Narayanan S, Kavanagh S, Mukherjee K, Balasubramanian V, Hosagrahara VP, Solapure S, Ravishankar S, Hameed PS (2017) Discovery of Imidazo[1,2-a]pyridine Ethers and Squaramides as Selective and Potent Inhibitors of Mycobacterial Adenosine Triphosphate (ATP) Synthesis. J Med Chem 60(4):1379–1399. https://doi.org/10.1021/acs.jmedchem.6b01358
Foo CS, Lupien A, Kienle M, Vocat A, Benjak A, Sommer R, Lamprecht DA, Steyn AJC, Pethe K, Piton J, Altmann KH, Cole ST (2018) arylvinylpiperazine amides, a new class of potent inhibitors targeting qcrb of Mycobacterium tuberculosis. Bio 9(5):e01276-01218. https://doi.org/10.1128/mBio.01276-18
Cleghorn LAT, Ray PC, Odingo J, Kumar A, Wescott H, Korkegian A, Masquelin T, Lopez Moure A, Wilson C, Davis S, Huggett M, Turner P, Smith A, Epemolu O, Zuccotto F, Riley J, Scullion P, Shishikura Y, Ferguson L, Rullas J, Guijarro L, Read KD, Green SR, Hipskind P, Parish T, Wyatt PG (2018) Identification of Morpholino Thiophenes as Novel Mycobacterium tuberculosis Inhibitors. Targeting QcrB J Med Chem 61(15):6592–6608. https://doi.org/10.1021/acs.jmedchem.8b00172
Harrison GA, Mayer Bridwell AE, Singh M, Jayaraman K, Weiss LA, Kinsella RL, Aneke JS, Flentie K, Schene ME, Gaggioli M, Solomon SD, Wildman SA, Meyers MJ, Stallings CL (2019) Identification of 4-Amino-Thieno[2,3-d]Pyrimidines as QcrB Inhibitors in Mycobacterium tuberculosis. mSphere 4 (5): e00606–00619. https://doi.org/10.1128/mSphere.00606-19
Chong SMS, Manimekalai MSS, Sarathy JP, Williams ZC, Harold LK, Cook GM, Dick T, Pethe K, Bates RW, Gruber G (2020) Antituberculosis Activity of the Antimalaria Cytochrome bcc Oxidase Inhibitor SCR0911. ACS Infect Dis 6(4):725–737. https://doi.org/10.1021/acsinfecdis.9b00408
Amporndanai K, Johnson RM, O’Neill PM, Fishwick CWG, Jamson AH, Rawson S, Muench SP, Hasnain SS, Antonyuk SV (2018) X-ray and cryo-EM structures of inhibitor-bound cytochrome bc1 complexes for structure-based drug discovery. IUCrJ 5(Pt 2):200–210. https://doi.org/10.1107/S2052252518001616
Lupien A, Foo CS, Savina S, Vocat A, Piton J, Monakhova N, Benjak A, Lamprecht DA, Steyn AJC, Pethe K, Makarov VA, Cole ST (2020) New 2-Ethylthio-4-methylaminoquinazoline derivatives inhibiting two subunits of cytochrome bc1 in Mycobacterium tuberculosis. PLoS Pathog 16(1):e1008270. https://doi.org/10.1371/journal.ppat.1008270
Satish S, Chitral R, Kori A, Sharma B, Puttur J, Khan AA, Desle D, Raikuvar K, Korkegian A, Martis EAF, Iyer KR, Coutinho EC, Parish T, Nandan S (2021) Design, synthesis and SAR of antitubercular benzylpiperazine ureas. Mol. Divers.:1–24. https://doi.org/10.1007/s11030-020-10158-3
Wang A, Wang H, Geng Y, Fu L, Gu J, Wang B, Lv K, Liu M, Tao Z, Ma C, Lu Y (2019) Design, synthesis and antimycobacterial activity of less lipophilic Q203 derivatives containing alkaline fused ring moieties. Bioorg Med Chem 27(5):813–821. https://doi.org/10.1016/j.bmc.2019.01.022
Sellamuthu S, Bhat MF, Kumar A, Singh SK (2018) Phenothiazine: A Better Scaffold against Tuberculosis. Mini Rev Med Chem 18(17):1442–1451. https://doi.org/10.2174/1389557517666170220152651
Poce G, Cocozza M, Consalvi S, Biava M (2014) SAR analysis of new anti-TB drugs currently in pre-clinical and clinical development. Eur J Med Chem 86:335–351. https://doi.org/10.1016/j.ejmech.2014.08.066
de Jager VR, Dawson R, van Niekerk C, Hutchings J, Kim J, Vanker N, van der Merwe L, Choi J, Nam K, Diacon AH (2020) Telacebec (Q203), a New Antituberculosis Agent. N Engl J Med 382(13):1280–1281. https://doi.org/10.1056/NEJMc1913327
Kang S, Kim YM, Jeon H, Park S, Seo MJ, Lee S, Park D, Nam J, Lee S, Nam K, Kim S, Kim J (2017) Synthesis and structure–activity relationships of novel fused ring analogues of Q203 as antitubercular agents. Eur J Med Chem 136:420–427. https://doi.org/10.1016/j.ejmech.2017.05.021
Kang S, Kim YM, Kim RY, Seo MJ, No Z, Nam K, Kim S, Kim J (2017) Synthesis and structure–activity studies of side chain analogues of the anti-tubercular agent, Q203. Eur J Med Chem 125:807–815. https://doi.org/10.1016/j.ejmech.2016.09.082
Kang S, Kim RY, Seo MJ, Lee S, Kim YM, Seo M, Seo JJ, Ko Y, Choi I, Jang J, Nam J, Park S, Kang H, Kim HJ, Kim J, Ahn S, Pethe K, Nam K, No Z, Kim J (2014) Lead optimization of a novel series of imidazo[1,2-a]pyridine amides leading to a clinical candidate (Q203) as a multi- and extensively-drug-resistant anti-tuberculosis agent. J Med Chem 57(12):5293–5305. https://doi.org/10.1021/jm5003606
Li L, Li Z, Liu M, Shen W, Wang B, Guo H, Lu Y (2015) Design, Synthesis and antimycobacterial activity of novel imidazo[1,2-a]pyridine amide-cinnamamide hybrids. Molecules 21(1):E49. https://doi.org/10.3390/molecules21010049
Wang A, Lv K, Li L, Liu H, Tao Z, Wang B, Liu M, Ma C, Ma X, Han B, Wang A, Lu Y (2019) Design, synthesis and biological activity of N-(2-phenoxy)ethyl imidazo[1,2-a]pyridine-3-carboxamides as new antitubercular agents. Eur J Med Chem 178:715–725. https://doi.org/10.1016/j.ejmech.2019.06.038
Wang H, Wang A, Gu J, Fu L, Lv K, Ma C, Tao Z, Wang B, Liu M, Guo H, Lu Y (2019) Synthesis and antitubercular evaluation of reduced lipophilic imidazo[1,2-a]pyridine-3-carboxamide derivatives. Eur J Med Chem 165:11–17. https://doi.org/10.1016/j.ejmech.2018.12.071
Yanofsy DJ, Di Trani JM, Krol S, Abdelaziz R, Bueler SA, Imming P, Brzezinski P, Rubinstein JL (2021) Structure of mycobacterial CIII2CIV2 respiratory supercomplex bound to the tuberculosis drug candidate telacebec (Q203). eLife 10. https://doi.org/10.7554/eLife.71959
Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281(5373):64–71. https://doi.org/10.1126/science.281.5373.64
Kim H, **a D, Yu CA, **a JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J (1998) Inhibitor binding changes domain mobility in the iron-sulfur protein of the mitochondrial bc1 complex from bovine heart. Proc Natl Acad Sci U S A 95(14):8026–8033. https://doi.org/10.1073/pnas.95.14.8026
Mulkidjanian AY (2005) Ubiquinol oxidation in the cytochrome bc1 complex: reaction mechanism and prevention of short-circuiting. Biochim Biophys Acta 1709(1):5–34. https://doi.org/10.1016/j.bbabio.2005.03.009
Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH (1998) Electron transfer by domain movement in cytochrome bc1. Nature 392(6677):677–684. https://doi.org/10.1038/33612
Darrouzet E, Moser CC, Dutton PL, Daldal F (2001) Large scale domain movement in cytochrome bc1: a new device for electron transfer in proteins. Trends in Biochem Sci 26(7):445–451. https://doi.org/10.1016/s0968-0004(01)01897-7
Berry EA, De Bari H (1827) Huang LS (2013) Unanswered questions about the structure of cytochrome bc1 complexes. Biochim Biophys Acta 11–12:1258–1277. https://doi.org/10.1016/j.bbabio.2013.04.006
Sodero AC, Abrahim-Vieira B, Torres PH, Pascutti PG, Garcia CR, Ferreira VF, Rocha DR, Ferreira SB, Silva FP Jr (2017) Insights into cytochrome bc1 complex binding mode of antimalarial 2-hydroxy-1,4-naphthoquinones through molecular modelling. Mem Inst Oswaldo Cruz 112(4):299–308. https://doi.org/10.1590/0074-02760160417
Ko Y, Choi I (2016) Putative 3D structure of QcrB from Mycobacterium tuberculosis cytochromebc1 complex, a novel drug-target for new series of antituberculosis agent Q203. Bull Korean Chem Soc 37(5):725–731. https://doi.org/10.1002/bkcs.10765
McConnell EV, Bruzual I, Pou S, Winter R, Dodean RA, Smilkstein MJ, Krollenbrock A, Nilsen A, Zakharov LN, Riscoe MK, Doggett JS (2018) Targeted structure–activity analysis of endochin-like quinolones reveals potent Qi and Qo site inhibitors of toxoplasma gondii and plasmodium falciparum cytochrome bc1 and identifies ELQ-400 as a remarkably effective compound against acute experimental toxoplasmosis. ACS Infect Dis 4(11):1574–1584. https://doi.org/10.1021/acsinfecdis.8b00133
Nixon GL, Pidathala C, Shone AE, Antoine T, Fisher N, O’Neill PM, Ward SA, Biagini GA (2013) Targeting the mitochondrial electron transport chain of Plasmodium falciparum: new strategies towards the development of improved antimalarials for the elimination era. Future Med Chem 5(13):1573–1591. https://doi.org/10.4155/fmc.13.121
Lucumi E, Darling C, Jo H, Napper AD, Chandramohanadas R, Fisher N, Shone AE, **g H, Ward SA, Biagini GA, DeGrado WF, Diamond SL, Greenbaum DC (2010) Discovery of potent small-molecule inhibitors of multidrug-resistant Plasmodium falciparum using a novel miniaturized high-throughput luciferase-based assay. Antimicrob Agents Chemother 54(9):3597–3604. https://doi.org/10.1128/AAC.00431-10
Zhao PL, Wang L, Zhu XL, Huang X, Zhan CG, Wu JW, Yang GF (2010) Subnanomolar inhibitor of cytochrome bc1 complex designed by optimizing interaction with conformationally flexible residues. J Am Chem Soc 132(1):185–194. https://doi.org/10.1021/ja905756c
Hao GF, Wang F, Li H, Zhu XL, Yang WC, Huang LS, Wu JW, Berry EA, Yang GF (2012) Computational discovery of picomolar Q(o) site inhibitors of cytochrome bc1 complex. J Am Chem Soc 134(27):11168–11176. https://doi.org/10.1021/ja3001908
Abdullahi M, Adeniji SE, Arthur DE, Haruna A (2021) Homology modeling and molecular docking simulation of some novel imidazo[1,2-a]pyridine-3-carboxamide (IPA) series as inhibitors of Mycobacterium tuberculosis. J Genet Eng Biotechnol 19(1):1–12. https://doi.org/10.1186/s43141-020-00102-1
Silva DR, Dalcolmo M, Tiberi S, Arbex MA, Munoz-Torrico M, Duarte R, D’Ambrosio L, Visca D, Rendon A, Gaga M, Zumla A, Migliori GB (2018) New and repurposed drugs to treat multidrug—and extensively drug-resistant tuberculosis. J Bras Pneumol 44(2):153–160. https://doi.org/10.1590/s1806-37562017000000436
Migliori GB, Besozzi G, Girardi E, Kliiman K, Lange C, Toungoussova OS, Ferrara G, Cirillo DM, Gori A, Matteelli A, Spanevello A, Codecasa LR, Raviglione MC, Group STS (2007) Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur. Respir. J. 30 (4):623-626. https://doi.org/10.1183/09031936.00077307
Sacks LV, Behrman RE (2009) Challenges, successes and hopes in the development of novel TB therapeutics. Future Med Chem 1(4):749–756. https://doi.org/10.4155/fmc.09.53
Berube BJ, Russell D, Castro L, Choi SR, Narayanasamy P, Parish T (2019) Novel MenA Inhibitors Are Bactericidal against Mycobacterium tuberculosis and Synergize with Electron Transport Chain Inhibitors. Antimicrob Agents Chemother 63(6):e02661. https://doi.org/10.1128/AAC.02661-18
Kurosu M, Crick DC (2009) MenA is a promising drug target for develo** novel lead molecules to combat Mycobacterium tuberculosis. Med Chem 5(2):197–207. https://doi.org/10.2174/157340609787582882
Bahuguna A, Rawat DS (2020) An overview of new antitubercular drugs, drug candidates, and their targets. Med Res Rev 40(1):263–292. https://doi.org/10.1002/med.21602
Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A (2014) Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS ONE 9(7):e102135. https://doi.org/10.1371/journal.pone.0102135
Grossman TH, Shoen CM, Jones SM, Jones PL, Cynamon MH, Locher CP (2015) The efflux pump inhibitor timcodar improves the potency of antimycobacterial agents. Antimicrob Agents Chemother 59(3):1534–1541. https://doi.org/10.1128/AAC.04271-14
Small JL, Park SW, Kana BD, Ioerger TR, Sacchettini JC, Ehrt S (2013) Perturbation of cytochrome c maturation reveals adaptability of the respiratory chain in Mycobacterium tuberculosis. MBio 4(5):e00475-00413. https://doi.org/10.1128/mBio.00475-13
Lamprecht DA, Finin PM, Rahman MA, Cumming BM, Russell SL, Jonnala SR, Adamson JH, Steyn AJ (2016) Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun 7(1):12393. https://doi.org/10.1038/ncomms12393
Arora K, Ochoa-Montano B, Tsang PS, Blundell TL, Dawes SS, Mizrahi V, Bayliss T, Mackenzie CJ, Cleghorn LA, Ray PC, Wyatt PG, Uh E, Lee J, Barry CE 3rd, Boshoff HI (2014) Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58(11):6962–6965. https://doi.org/10.1128/AAC.03486-14
Acknowledgements
The authors would like to thank NIPER Kolkata for providing the resources and support. We would like to acknowledge the Department of Pharmaceuticals and the Ministry of Chemicals and Fertilizes for providing the fellowship to Mr. Wani.
Funding
This manuscript was not funded.
Author information
Authors and Affiliations
Contributions
MAW collected the literature and wrote the manuscript. DKD revised and approved the final manuscript submitted for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wani, M.A., Dhaked, D.K. Targeting the cytochrome bc1 complex for drug development in M. tuberculosis: review. Mol Divers 26, 2949–2965 (2022). https://doi.org/10.1007/s11030-021-10335-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10335-y