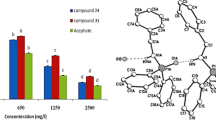
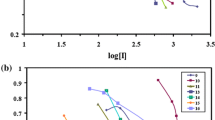
Abstract
In an attempt to obtain the modified and novel insecticides with low human toxicity, a series of novel mono-, bis-, and tetraphosphonic acid derivatives were designed and characterized by infrared, 1H, 13C, and 31P NMR spectroscopy and X-ray crystallography. The inhibitory effects of the synthesized compounds were evaluated using the in vitro Ellman method on human and insect acetylcholinesterase (AChE). Some of these compounds, which had low human and high insect toxicity, were chosen to assess the killing effects (in vivo) on third larval instar of elm leaf beetle (X. luteola). In vivo and in vitro evidence has revealed that bisphosphonic acids, containing hydrophobic systems, have a good selectivity of insect AChE inhibition. In the present study, docking results showed that bisphosphonic acids had lower binding energy and higher inhibition compared with tetraphosphonic acids due to the type of their topology and the ability of their hydrogen to interact with the catalytic triad (the main active site of the enzyme). Additionally, the QSAR results demonstrated that the major effecting factors on the insecticidal activity of the subject compounds are the hydrophobicity, size, shape, and ability to form a hydrogen bond. The present study can be helpful in the development of new insecticides.
Graphic abstract









Similar content being viewed by others
References
Rechcigl JE, Rechcigl NA (2016) Insect pest management: techniques for environmental protection. CRC Press, London
Casida JE, Quistad GB (2004) Chem Res Toxicol 17:983–998
Sparks TC (2013) Pestic Biochem Physiol 107:8–17
King AM, Aaron CK (2015) Emerg Med Clin 33:133–151
N. Zerangue, Q. Gao and W. J. Dower (2010) Creatine phosphate analog prodrugs, compositions and uses thereof, Google Patents
Bandyopadhyay P, Sathe M, Tikar SN, Yadav R, Sharma P, Kumar A, Kaushik M (2014) Bioorg Med Chem Lett 24:2934–2939
Gao J-R, Zhu KY (2001) Insect Biochem Mol Biol 31:1095–1104
Gholivand K, Mohammadpanah F, Pooyan M, Valmoozi AAE, Sharifi M, Mani-Varnosfaderani A, Hosseini Z (2019) Pestic Biochem Physiol 157:122–137
Naydenova ED, Todorov PT, Troev KD (2010) Amino Acids 38:23–30
Alcocer M, Dillon P, Manning B, Doyen C, Lee H, Daly S, O’kennedy R, Morgan M (2000) J Agric Food Chem 48:2228–2233
Molina M, Silva M (2002) Electrophoresis 23:2333–2340
Tsebrikova G, Baulin V, Kalashnikova I, Ragulin V, Zavel’skii V, Maruk AY, Lunev A, Klement’eva O, Kodina G, Tsivadze AY (2015) Russ J Gen Chem 85:2071–2079
Wagner-Jauregg T, Hackley BE Jr, Lies T, Owens O, Proper R (1955) J Am Chem Soc 77:922–929
Yu H, Yang H, Shi E, Tang W (2020) Med Drug Discov 8:100063
Lountos GT, Tropea JE, Waugh DS (2013) Mol Biochem Parasitol 187:1–8
Zhang Z, He D, Chen R (1997) Phosphorus Sulfur Silicon Relat Elem 126:273–281
Gholivand K, Ghaziani F, Yaghoubi R, Hosseini Z, Shariatinia Z (2010) J Enzyme Inhib Med Chem 25:827–835
Sedlak J, Lindsay RH (1968) Anal Biochem 25:192–205
R. Eisenthal and M. J. Danson, (2002) Enzyme assays: a practical approach, Practical Approach (Paperback)
Bashari E, Ghadamyari M, Sendi JJ (2014) J Entomol Soc Iran 34:35–46
Chauhan MS, Shukla J, Pandey U, Bhadauria S (2013) Int J Res Bot 3:37–43
L. Software, (1987) A user's guide to probit and logit analysis. LeOra Software Company, Berkeley, CA
Gholivand K, Shariatinia Z, Khajeh K, Naderimanesh H (2006) J Enzyme Inhib Med Chem 21:31–35
Gwaram NS, Ali HM, Abdulla MA, Buckle MJ, Sukumaran SD, Chung LY, Othman R, Alhadi AA, Yehye WA, Hadi AHA (2012) Molecules 17:2408–2427
Casida JE, Durkin KA (2013) Chem Biol Interact 203:221–225
Todeschini R, Consonni V (2008) Handbook of molecular descriptors. Wiley, London
Jalali-Heravi M, Fatemi M (2001) J Chromatogr A 915:177–183
Gholivand K, Pooyan M, Mohammadpanah F, Pirastefar F, Junk PC, Wang J, Valmoozi AAE, Mani-Varnosfaderani A (2019) Bioorg Chem 86:482–493
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Adv Drug Deliv Rev 23:3–25
Acknowledgements
We would like to thank our colleagues from the University of Tarbiat Modares who provided valuable insights.
Funding
This work was supported by the University of Tarbiat Modares.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. And all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gholivand, K., Mohammadpanah, F., Yaghoubi, R. et al. Synthesis, crystal structure, cholinesterase inhibitory activity, evaluation of insecticidal activities, and computational studies of new phosphonic acids. Mol Divers 26, 1519–1530 (2022). https://doi.org/10.1007/s11030-021-10283-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10283-7