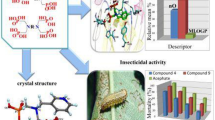
Abstract
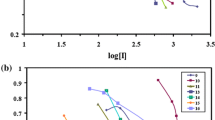
Synthesizing new chemical compounds and studying their biological applications have been important issues in scientific research. In this investigation, we synthesized and characterized ten new N-acetyl phosphoramidate compounds and explored the crystal structure of three others. Furthermore, not only were some kinetic inhibition parameters measured, like IC50, Ki, kp, KD for 7 compounds on human acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), but also their hydrophobic parameter was determined by shake-flask technique. All compounds (number 1–10) were investigated for anti-bacterial activity against three Gram-positive and three Gram-negative bacteria, while chloramphenicol was used as a standard antibiotic. In order to find new insecticide, toxicities of 13 acephate (Ace)-derived compounds (number 20–32) were bioassayed on third larval instar of elm leaf beetle and Xanthogaleruca luteola. Additionally, screening in vivo tests revealed that two compounds had had the greatest insecticidal potential in comparison with others. It means these ones inhibited AChE (with mixed mechanisms) and general esterase more than the rest. According to ChE-QSAR models, the inhibitory potency for enzyme and bacteria is directly influenced by the electronic parameters versus structural descriptors. AChE-QSPR model of fluorescence assay indicated that the inhibitory power of AChE is primarily influenced by a set of electronic factors with the priority of: EHB > PL > δ(31P) versus structural descriptor (SA and Mv).
Graphic abstract
Synthesizing new chemical compounds and studying their biological applications have been important issues in scientific research. Toxicities of 13 acephate (Ace)-derived compounds (number 20–32) were bioassayed on third larval instar of elm leaf beetle and Xanthogaleruca luteola. Insect-QSAR equations of these compounds, based on MLR and PCA, showed that non-descriptor net charge nitrogen atom (which was affected by the polarization of N–H group) had the greatest effect on insecticidal potential.






Similar content being viewed by others
References
Gholivand K, Shariatinia Z, Khajeh K, Naderi-Manesh H (2006) Synthesis and spectroscopic characterization of some phosphoramidates as reversible inhibitors of human acetylcholinesterase and determination of their potency. J Enzyme Inhib Med Chem 21:31–35
Gholivand K, Ghaziani F, Yaghoubi R, Hosseini Z, Shariatinia Z (2010) Design, synthesis and anticholinesterase activity of some new α-aminobisphosphonates. J Enzyme Inhib Med Chem 25:827–835
Vadapalli C, Ramachandran A, Stefano Z, Jamie B, Alexander S (2005) Synthesis, structure, and stereochemistry of trinuclear metal complexes formed from the phosphorus-based achiral tripodal ligand P(S)[N(Me)N = CHC6H4-o-OH]3 (LH3): luminescent properties of L2Cd3·2H2O. Inorg Chem 44:4608–4615
Chandrasekhar V, Azhakar R, Bickley J, Steiner A (2006) Influence of O–H···O=P hydrogen bonding on the supramolecular architectures of phosphorus-based hydrazones: alternate right- and left-handed fused helical chains based on O–H·O=P hydrogen bonds in the crystal structure of C6H5P(O)[N(CH3) N = CHC6H4-p-OH]2. Cryst Growth Des 6:910–914
Harger M (2001) Phosphorochloridates from phosphorohydrazides with ButOCl: stereospecificity, selectivity and phosphorylium ion intermediates. Tetra Lett 42:6749–6752
Blower P (2004) Inorganic pharmaceuticals. Annu Rep Prog Chem Sect A Inorg Chem 100:633–658
Casida JE, Quistad GB (2004) Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol 17:983–998
Sparks TC (2013) Insecticide discovery: an evaluation and analysis. Pest Biochem Physiol 17:8–17
Benfenati E (2007) Quantitative structure–activity relationships (QSAR) for pesticide regulatory purposes. Elsevier B.V., New York
Ellman GL, Courthey KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Gholivand K, Hosseini Z, Farshadian S, Naderi-Manesh H (2010) Synthesis, characterization, oxidative degradation, antibacterial activity and acetylcholinesterase/butyrylcholinesterase inhibitory effects of some new phosphorus(V) hydrazides. Eur J Med Chem 45:5130–5139
Gholivand K, Ebrahimi Valmoozi AA, Dashtaki MR, Mohamadpanah F et al (2017) Synthesis, crystal structure, molecular docking and QSAR/QSPR studies of Temephos derivatives as human and insect cholinesterase inhibitors. ChemistrySelect 2:8828–8840
Turk T, Macek P, Suput D (1995) Inhibition of acetylcholinesterase by a pseudozoanthoxanthin-like compound isolated from the zoanthid Parazoanthus axinellae (O. Schmidt). Toxicon 33:133–142
Gould J, Bowie J (1952) The determination of bacterial sensitivity to antibiotics. Edinb Med J 59:178–199
Bashari E, Ghadamyari M, Sendi JJ (2014) Toxicity and biological and biochemical effects of hexaflumuron on the elm leaf beetle, Xanthogaleruca luteola (Col.: chrysomelidae). J Entomol Soc Iran 34:46–59
Chauhan MS, Shukla JP, Pandey UK, Bhadauria S (2013) Efficacy of some plant products as repellent to control Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) fed on Tomato (Lycopersicon esculentum). Int J Res Bot 3:37–43
Van Asperen E (1962) A study of housefly esterase by mean of a sensitive colorimetric method. J Insect Physiol 8:401–416
Gao JR, Zhu KY (2001) an acetylcholinesterase purified from the greenbug (Schizaphis graminum) with unique enzymological and pharmacological characteristics. Insect Biochem Mol Biol 31:1095–1104
Eisenthal M, Danson M (2002) Enzyme assays: a practical approach (practical approach series), 2nd edn. Oxford University Press, Oxford
Schultz T, Cronin M, Walker J, Aptula A (2003) Quantitative structure–activity relationships (QSARs) in toxicology: a historical perspective. J Mol Struct (Theochem) 622:1–22
SAS Institute (2002) SAS/GRAPH software: reference volume 2 Version 8. SAS Institute Inc., Cary
Hansch C, Fujita T (1964) A method for the correlation of biological activity and chemical structure. J Am Chem Soc 86:1616–1626
SPSS for Windows, Version 10.05, SPSS Inc., Bangalore, India (1999)
Schuurmann G, Ebert RU, Chen JW, Wang B, Kuhne R (2008) External validation and prediction employing the predictive squared correlation coefficient test set activity mean vs training set activity mean. J Chem Inf Model 48:2140–2145
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck K, Raghavachari AD, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2005) Gaussian 03, Revision D.01. Gaussian, Inc., Wallingford
Gholivand K, Pourayoubi M, Shariatinia Z (2007) 2,3J(P, X) [X = H, C] coupling constants dependency on the ring size, hybridization and substituents in new diazaphospholes and diazaphosphorinanes, NMR and X-ray crystallography studies. Polyhedron 26:837–844
Gholivand K, Hosseini Z, Pourayoubi M, Shariatinia Z (2005) Synthesis and spectroscopic study of some new, crystal structures of N-Benzoyl-N, N-bis(azetidinyl)phosphoric triamide and N-Benzoyl-N, N-bis(hexamethylenyl)phosphoric triamide. Z Anorg Allg Chem 631:3074–3079
Gholivand K, Pourayoubi M, Shariatinia Z, Mostaanzadeh H (2005) The effect of various substituents on the structural parameters of the P(O)[N(CH3)(CH2C6H5)]2 moieties. Syntheses and spectroscopic characterization of some new phosphoramidates, crystal structures of P(O)(X)[N(CH3)(CH2C6H5)]2, X = C6H5C(O)NH, Cl and CCl3C(O)NH. Polyhedron 24:655–662
Gholivand K, Mojahed F, Madani Alizadehgan A (2007) Synthesis and characterization of novel phosphorictriamide derivatives with morpholine substituents: crystal structures of (CF3)C(O)NHP(O)(NC4H8O)2 and (p-Br-C6H4)C(O)NHP(O)(NC4H8O)2. Polish J Chem 81:393–402
Segel I (1975) Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley, New York
Copeland RA (2000) Enzymes, a practical introduction to structure, mechanism, and data analysis, 2nd edn. Wiley-VCH, New York
Main A (1964) Affinity and phosphorylation constants for the inhibition of esterases by organophosphates. Science 144:992–993
Roy DR, Sarkar U, Chattaraj PK, Mitra A, Padmanabhan J, Parthasarathi R, Subramanian V, Van Damme S, Bultinck P (2006) Analyzing toxicity through electrophilicity. Mol Divers 10:119–131
Riazzo S, Riviere C, Piazzi L, Bisi A, Gobbi S, Bartolini M, Andrisano V, Morroni F, Tarozzi A, Monti J, Rampa A (2008) Benzofuran-based hybrid compounds for the inhibition of cholinesterase activity, betaamyloid aggregation, and abeta neurotoxicity. J Med Chem 51:2883–2886
Singh J, Shaik B, Singh S, Agrawal VK, Khadikar PV, Deeb O, Supuran CT (2008) Comparative QSAR study on para-substituted aromatic sulphonamides as CAII inhibitors: information versus topological (distance-based and connectivity) indices. Chem Biol Drug Des 71:244–259
Asadi L, Gholivand K, Zare K (2016) Phosphorhydrazides as urease and acetylcholinesterase inhibitors: biological evaluation and QSAR study. J Iran Chem Soc 3:12
Gholivand K, Valmoozi AAE, Mahzouni HR, Ghadimi S, Rahimi R (2013) Molecular docking and QSAR studies: noncovalent interaction between acephate analogous and the receptor site of human acetylcholinesterase. J Agric Food Chem 6:6776
Gholivand K, Mohammadpanah F, Pooyan M, Valmoozi AAE, Sharifi M, Mani-Varnosfaderani A, Hosseini Z (2019) Synthesis, crystal structure, insecticidal activities, molecular docking and QSAR studies of some new phospho guanidines and phospho pyrazines as cholinesterase inhibitors. Pestic Biochem Physiol 157:122–137
Acknowledgements
Financial support of this work by Iran National Science Foundation: INSF and Tarbiat Modares University, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gholivand, K., Roshanian, Z., Rahimzadeh Dashtaki, M. et al. Monophosphoramide derivatives: synthesis and crystal structure, theoretical and experimental studies of their biological effects. Mol Divers 26, 97–112 (2022). https://doi.org/10.1007/s11030-020-10160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10160-9