Abstract
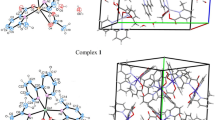
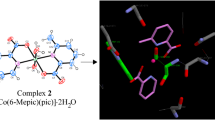
The World Health Organization (WHO) report shows that diabetes mellitus (DM) will be one of the ten deadly diseases in the near future. The best way to prevent DM is to decrease blood glucose levels and keep under control; therefore, it is important to design and synthesize the effective inhibitors that can be used in the treatment of DM disease. In this respect, a series of ten metal complexes containing 6-methylpyridine-2-carboxylic acid {[Cr(6-mpa)2(H2O)2]·H2O·NO3, (1), [Mn(6-mpa)2(H2O)2], (2), [Ni(6-mpa)2(H2O)2]·2H2O, (3), [Hg(6-mpa)2(H2O)], (4), [Cu(6-mpa)2(Py)], (5), [Cu(6-mpa)2(H2O)]·H2O, (6), [Zn(6-mpa)2(H2O)]·H2O, (7), [Fe(6-mpa)3], (8), [Cd(6-mpa)2(H2O)2]·2H2O, (9), and [Co(6-mpa)2(H2O)2]·2H2O, (10)} were synthesized as α-glucosidase inhibitors. We found that the IC50 values of the synthesized complexes ranged from 0.247 ± 0.10 to > 600 μM against α-glucosidase. The spectral analyses for these complexes characterized by XRD and LC–MS/MS were also carried out by FT-IR and UV–Vis spectra. Additionally, the DFT/HSEh1PBE/6-311G(d,p)/LanL2DZ level was applied to obtain optimal molecular geometries and spectral behaviors as well as significant contributions to the electronic transitions for the complexes. The molecular docking study was also performed to display interactions between the target protein (the template structure Saccharomyces cerevisiae isomaltase) and the synthesized complexes (1–10).
Graphic abstract










Similar content being viewed by others
Change history
17 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11030-021-10299-z
References
Cai C-Y, Rao L, Rao Y, Guo J-X, **ao Z-Z, Cao J-Y, Huang Z-S, Wang B (2017) Analogues of xanthones—chalcones and bis-chalcones as α-glucosidase inhibitors and anti-diabetes candidates. Eur J Med Chem 130:51–59. https://doi.org/10.1016/j.ejmech.2017.02.007
Zhen J, Dai Y, Villani T, Giurleo D, Simon JE, Wu Q (2017) Synthesis of novel flavonoid alkaloids as α-glucosidase inhibitors. Bioorg Med Chem 25(20):5355–5364. https://doi.org/10.1016/j.bmc.2017.07.055
Ghani U (2015) Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: finding needle in the haystack. Eur J Med Chem 103:133–162. https://doi.org/10.1016/j.ejmech.2015.08.043
Adisakwattana S, Charoenlertkul P, Yibchok-anun S (2009) α-Glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. J Enzym Inhib Med Chem 24(1):65–69. https://doi.org/10.1080/14756360801906947
Chiasson J, Josse R, Gomis R, Hanefeld M, Karasik A, Laakso M (2004) STOP-NIDDM Trial Research Group: acarbose for the prevention of type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia 47(6):969–975. https://doi.org/10.1016/S0140-6736(02)08905-5
Santos CM, Freitas M, Fernandes E (2018) A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur J Med Chem 157:1460–1479. https://doi.org/10.1016/j.ejmech.2018.07.073
Wang Y, Ma L, Li Z, Du Z, Liu Z, Qin J, Wang X, Huang Z, Gu L, Chen AS (2004) Synergetic inhibition of metal ions and genistein on α-glucosidase. FEBS Lett 576(1–2):46–50. https://doi.org/10.1016/j.febslet.2004.08.059
Yoshikawa Y, Hirata R, Yasui H, Sakurai H (2009) Alpha-glucosidase inhibitory effect of anti-diabetic metal ions and their complexes. Biochimie 91(10):1339–1341. https://doi.org/10.1016/j.biochi.2009.06.005
Avcı D, Altürk S, Sönmez F, Tamer Ö, Başoğlu A, Atalay Y, Kurt BZ, Dege N (2018) Three novel Cu (II), Cd (II) and Cr(III) complexes of 6 − Methylpyridine − 2 − carboxylic acid with thiocyanate: synthesis, crystal structures, DFT calculations, molecular docking and α-Glucosidase inhibition studies. Tetrahedron 74(50):7198–7208. https://doi.org/10.1016/j.tet.2018.10.054
Avcı D, Altürk S, Sönmez F, Tamer Ö, Başoğlu A, Atalay Y, Zengin Kurt B, Dege N (2019) A novel series of M (II) complexes of 6-methylpyridine-2-carboxylic acid with 4 (5) methylimidazole: synthesis, crystal structures, α-glucosidase activity, density functional theory calculations and molecular docking. Appl Organomet Chem 33(7):e4935. https://doi.org/10.1002/aoc.4935
Avcı D, Altürk S, Sönmez F, Tamer Ö, Başoğlu A, Atalay Y, Zengin Kurt B, Öztürk D, Dege N (2019) A new dinuclear copper (II) complex of 2, 5–Furandicarboxyclic acid with 4 (5)-Methylimidazole as a high potential α-glucosidase inhibitor: synthesis, crystal structure, cytotoxicity study, and TD/DFT calculations. Appl Organomet Chem 33(3):e4725. https://doi.org/10.1002/aoc.4725
Zheng J, Ma L (2015) Silver (I) complexes of 2, 4-dihydroxybenzaldehyde–amino acid Schiff bases—novel noncompetitive α-glucosidase inhibitors. Bioorg Med Chem Lett 25(10):2156–2161. https://doi.org/10.1016/j.bmcl.2015.03.078
Avcı D, Altürk S, Sönmez F, Tamer Ö, Başoğlu A, Atalay Y, Kurt BZ, Dege N (2019) A novel series of mixed-ligand M (II) complexes containing 2, 2′-bipyridyl as potent α-glucosidase inhibitor: synthesis, crystal structure, DFT calculations, and molecular docking. JBIC, J Biol Inorg Chem 24(5):747–764. https://doi.org/10.1007/s00775-019-01688-9
Constable EC, Steel PJ (1989) N, N′-Chelating biheteroaromatic ligands; a survey. Coord Chem Rev 93(2):205–223. https://doi.org/10.1016/0010-8545(89)80016-5
Usman A, Fun H-K, Chantrapromma S, Zhang M, Chen Z-F, Tang Y-Z, Shi S-M, Liang H (2003) Diacetatobis (2-aminobenzothiazole) zinc (II). Acta Crystallogr E 59(1):m41–m43. https://doi.org/10.1107/S1600536802021864
Adams H, Bailey NA, Crane JD, Fenton DE, Latour J-M, Williams JM (1990) Manganese (II) and iron (III) complexes of the tridentate ligands bis (benzimidazol-2-ylmethyl)-amine (L 1) and-methylamine (L 2). Crystal structures of [MnL1(CH3CO2)2],[FeL2Cl3], and [Fe2 L12(µ-O){µ-(CH3)3CCO2}2][ClO4]2. J Chem Soc, Dalton Trans 5:1727–1735. https://doi.org/10.1039/DT9900001727
Driessen W, De Graaff R, Parlevliet F, Reedijk J, De Vos R (1994) Transition metal compounds of the tridentate pyrazole substituted amine ligand bis (2-(3,5-dimethyl-1-pyrazolyl) ethyl) ethylamine (ddae). X-ray structures of [Co(ddae)(NO3)2],[Cu(ddae)(NO3)(H2O)](NO3) and [Cu(ddae)(Cl)2]· C2H5OH. Inorg Chim Acta 216(1-2):43–49. https://doi.org/10.1016/0020-1693(93)03707-H
Gollapalli M, Taha M, Javid MT, Almandil NB, Rahim F, Wadood A, Mosaddik A, Ibrahim M, Alqahtani MA, Bamarouf YA (2019) Synthesis of benzothiazole derivatives as a potent α-glucosidase inhibitor. Bioorg Chem 85:33–48. https://doi.org/10.1002/1099-0682(200011)2000:11%3c2363:AID-EJIC2363%3e3.0.CO;2-M
Groß F, Müller-Hartmann A, Vahrenkamp H (2000) Diphosphate–Zinc complexes with tridentate coligands. Eur J Med Chem 11:2363–2370. https://doi.org/10.1016/j.bioorg.2018.12.021
Demadis KD, Katarachia SD (2004) Metal-phosphonate chemistry: synthesis, crystal structure of calcium-amino tris-(methylene phosphonate) and inhibition of CaCO3 crystal growth. Phosphorus, Sulfur Silicon Relat Elem 179(3):627–648. https://doi.org/10.1080/10426500490441514
Mao J-G (2007) Structures and luminescent properties of lanthanide phosphonates. Coord Chem Rev 251(11–12):1493–1520. https://doi.org/10.1016/j.ccr.2007.02.008
Monot J, Petit M, Lane SM, Guisle I, Léger J, Tellier C, Talham DR, Bujoli B (2008) Towards zirconium phosphonate-based microarrays for probing DNA–protein interactions: critical influence of the location of the probe anchoring groups. J Am Chem Soc 130(19):6243–6251. https://doi.org/10.1021/ja711427q
Hardy AM, LaDuca RL (2009) Synthesis and structure of a cobalt dicyanamide chain coordination polymer incorporating a long-spanning hydrogen-bonding capable diimine with a novel binodal (4, 6)-connected supramolecular topology. Inorg Chem Commun 12(4):308–311. https://doi.org/10.1016/j.inoche.2009.02.002
Umeda J, Suzuki M, Kato M, Moriya M, Sakamoto W, Yogo T (2010) Proton conductive inorganic–organic hybrid membranes functionalized with phosphonic acid for polymer electrolyte fuel cell. J Power Sources 195(18):5882–5888. https://doi.org/10.1016/j.jpowsour.2009.12.078
Saito Y, Takemoto J, Hutchinson B, Nakamoto K (1972) Infrared studies of coordination compounds containing low-oxidation-state metals. I. Tris (2, 2′-bipyridine) and tris (1, 10-phenanthroline) complexes. Inorg Chem 11(9):2003–2011. https://doi.org/10.1021/ic50115a004
Amani V, Safari N, Khavasi HR (2007) Synthesis, characterization and crystal structure determination of iron (III) hetero-ligand complexes containing 2, 2′-bipyridine, 5, 5′-dimethyl-2, 2′-bipyridine and chloride,[Fe (bipy) Cl4][bipy· H] and [Fe (dmbipy) 2Cl2][FeCl4]. Polyhedron 26(15):4257–4262. https://doi.org/10.1016/j.poly.2007.05.050
Mobin SM, Saini AK, Mishra V, Chaudhary A (2016) A series of new heteroleptic Hg(II) complexes: synthesis, crystal structures and photophysical properties. Polyhedron 110:131–141. https://doi.org/10.1016/j.poly.2016.02.037
Shechter Y, Karlish SJ (1980) Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature 284(5756):556. https://doi.org/10.1038/284556a0
Coulston L, Dandona P (1980) Insulin-like effect of zinc on adipocytes. Diabetes 29(8):665–667. https://doi.org/10.2337/diab.29.8.665
Sorenson JR (1989) 6 copper complexes offer a physiological approach to treatment of chronic diseases. Prog Med Chem 26:437–568. https://doi.org/10.1016/S0079-6468(08)70246-7
Anderson RA, Cheng N, Bryden NA, Polansky MM, Cheng N, Chi J, Feng J (1997) Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 46(11):1786–1791. https://doi.org/10.2337/diab.46.11.1786
Yasui H, Tamura A, Takino T, Sakurai H (2002) Structure-dependent metallokinetics of antidiabetic vanadyl-picolinate complexes in rats: studies on solution structure, insulinomimetic activity, and metallokinetics. J Inorg Biochem 91(1):327–338. https://doi.org/10.1016/S0162-0134(02)00443-9
Altürk S, Avcı D, Kurt BZ, Tamer Ö, Başoğlu A, Sönmez F, Atalay Y, Dege N (2019) Two new Co (II) complexes of picolinate: synthesis, crystal structure, spectral characterization, α-glucosidase inhibition and TD/DFT study. J Inorg Organomet Polym Mater 29(4):1265–1279. https://doi.org/10.1007/s10904-019-01090-7
Yasumatsu N, Yoshikawa Y, Adachi Y, Sakurai H (2007) Antidiabetic copper (II)-picolinate: impact of the first transition metal in the metallopicolinate complexes. Bioorg Med Chem 15(14):4917–4922. https://doi.org/10.1016/j.bmc.2007.04.062
Yoshikawa Y, Ueda E, Kawabe K, Miyake H, Takino T, Sakurai H, Kojima Y (2002) Development of new insulinomimetic zinc (II) picolinate complexes with a Zn (N2O2) coordination mode: structure characterization, in vitro, and in vivo studies. JBIC, J Biol Inorg Chem 7(1–2):68–73. https://doi.org/10.1007/s007750100266
Ueda E, Yoshikawa Y, Sakurai H, Kojima Y, Kajiwara NM (2005) In vitro alpha-glucosidase inhibitory effect of Zn (II) complex with 6-methyl-2-picolinmethylamide. Chem Pharm Bull 53(4):451–452. https://doi.org/10.1248/cpb.53.451
Kukovec B-M, Vaz PD, Popovic Z, Calhorda MJ, Furić K, Pavlović G, Linarić MR (2008) Pseudopolymorphism in nickel (II) complexes with 6-methylpicolinate. Synthesis, structural, spectroscopic, thermal, and density functional theory studies. Cryst Growth Des 8(9):3465–3473. https://doi.org/10.1021/cg800512k
Kukovec B-M, Popović Z, Kozlevčar B, Jagličić Z (2008) 3D supramolecular architectures of copper (II) complexes with 6-methylpicolinic and 6-bromopicolinic acid: synthesis, spectroscopic, thermal and magnetic properties. Polyhedron 27(18):3631–3638. https://doi.org/10.1016/j.poly.2008.09.011
Altürk S, Avcı D, Başoğlu A, Tamer Ö, Atalay Y, Dege N (2018) Copper (II) complex with 6-methylpyridine-2-carboxyclic acid: experimental and computational study on the XRD, FT-IR and UV–Vis spectra, refractive index, band gap and NLO parameters. Spectrochim Acta A 190:220–230. https://doi.org/10.1016/j.saa.2017.09.041
Pons J, March R, Rius J, Ros J (2004) Zinc complexes of 6-methyl-2-pyridinecarboxylic acid. Crystal structure of [Zn(MeC5H3NCOO)2(H2O)]·H2O. Inorg Chim Acta 357(12):3789–3792. https://doi.org/10.1016/j.ica.2004.03.058
Altürk S, Avcı D, Tamer Ö, Atalay Y, Şahin O (2016) A cobalt (II) complex with 6-methylpicolinate: synthesis, characterization, second-and third-order nonlinear optical properties, and DFT calculations. J Phys Chem Solids 98:71–80. https://doi.org/10.1016/j.jpcs.2016.06.008
Sheldrick GM (2015) SHELXT–ıntegrated space-group and crystal-structure determination. Acta Crystallogr A 71(1):3–8. https://doi.org/10.1107/S2053273314026370
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39(3):453–457. https://doi.org/10.1107/S002188980600731X
Spek AL (2009) Structure validation in chemical crystallography. Acta Crystallogr D 65(2):148–155. https://doi.org/10.1107/S090744490804362X
Sun H, Ding W, Song X, Wang D, Chen M, Wang K, Zhang Y, Yuan P, Ma Y, Wang R (2017) Synthesis of 6-hydroxyaurone analogues and evaluation of their α-glucosidase inhibitory and glucose consumption-promoting activity: development of highly active 5, 6-disubstituted derivatives. Bioorg Med Chem Lett 27(15):3226–3230. https://doi.org/10.1016/j.bmcl.2017.06.040
Frisch MJ, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G et al. (2009) Gaussian 09, Revision D. 01, Gaussian. Inc, Wallingford
Dennington R, Keith T, Millam J (2009) GaussView, version 5. Semichem Inc, Shawnee Mission, KS
Heyd J, Scuseria GE (2004) Efficient hybrid density functional calculations in solids: assessment of the Heyd–Scuseria–Ernzerhof screened Coulomb hybrid functional. J Chem Phys 121(3):1187–1192. https://doi.org/10.1063/1.1760074
Krukau AV, Vydrov OA, Izmaylov AF, Scuseria GE (2006) Influence of the exchange screening parameter on the performance of screened hybrid functionals. J Chem Phys 125(22):224106. https://doi.org/10.1063/1.2404663
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80(7):3265–3269. https://doi.org/10.1063/1.447079
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82(1):270–283. https://doi.org/10.1063/1.448799
Runge E, Gross EK (1984) Density-functional theory for time-dependent systems. Phys Rev Lett 52(12):997. https://doi.org/10.1103/PhysRevLett.52.997
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem Phys 55(1):117–129. https://doi.org/10.1016/0301-0104(81)85090-2
Glendening E, Reed A, Carpenter J, Weinhold F (1998) NBO Version 3.1. TCI, University of Wisconsin, Madison
Yang JM, Chen CC (2004) GEMDOCK: a generic evolutionary method for molecular docking. Proteins: Struct Funct Bioinf 55(2):288–304. https://doi.org/10.1002/prot.20035
Scott AP, Radom L (1996) Harmonic vibrational frequencies: an evaluation of Hartree − Fock, Møller − Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Chem Phys 100(41):16502–16513. https://doi.org/10.1021/jp960976r
Varsányi G (1974) Assignments for vibrational spectra of seven hundred benzene derivatives, vol 1. Halsted Press
Alizadeh R, Amani V (2016) Syntheses, crystal structures, and photoluminescence of three cadmium (II) coordination complexes based on bipyridine ligands with different positioned methyl substituents. Inorg Chim Acta 443:151–159. https://doi.org/10.1016/j.ica.2015.12.034
Belicchi-Ferrari M, Bisceglie F, Cavalieri C, Pelosi G, Tarasconi P (2007) Bis (triphenylphosphine) 4-fluorobenzaldehyde thiosemicarbazone copper (I): forcing chelation through oxoanions. Polyhedron 26(14):3774–3782. https://doi.org/10.1016/j.poly.2007.04.026
Gorelsky S (2014) SWizard Program Revision 4.5, University of Ottawa, Ottawa, Canada, 2010
Şişman İ, Başoğlu A (2016) Effect of Se content on the structural, morphological and optical properties of Bi2Te3 − ySey thin films electrodeposited by under potential deposition technique. Mat Sci Semicon Proc 54:57–64. https://doi.org/10.1016/j.mssp.2016.07.001
Yang W, Parr RG (1985) Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci 82(20):6723–6726. https://doi.org/10.1073/pnas.82.20.6723
Reed AE, Weinhold F (1983) Natural bond orbital analysis of near-Hartree–Fock water dimer. J Chem Phys 78(6):4066–4073. https://doi.org/10.1063/1.445134
Foster AJ, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102(24):7211–7218. https://doi.org/10.1021/ja00544a007
Šponer J, Hobza P (1996) DNA base amino groups and their role in molecular interactions: ab initio and preliminary density functional theory calculations. Int J Quant Chem 57(5):959–970
Gadre SR, Shrivastava IH (1991) Shapes and sizes of molecular anions via topographical analysis of electrostatic potential. J Chem Phys 94(6):4384–4390. https://doi.org/10.1063/1.460625
Chemla DS, Zysss J (1987) Nonlinear optical properties of organic molecules and crystals. Academic Press, Orlando
Kamada K, Ueda M, Nagao H, Tawa K, Sugino T, Shmizu Y, Ohta K (2000) Molecular design for organic nonlinear optics: polarizability and hyperpolarizabilities of furan homologues investigated by ab initio molecular orbital method. J Phys Chem A 104(20):4723–4734. https://doi.org/10.1021/jp993806y
Pierce BM (1989) A theoretical analysis of third-order nonlinear optical properties of linear polyenes and benzene. J Chem Phys 91(2):791–811. https://doi.org/10.1063/1.457132
Cheng LT, Tam W, Stevenson SH, Meredith GR, Rikken G, Marder SR (1991) Experimental investigations of organic molecular nonlinear optical polarizabilities. 1. Methods and results on benzene and stilbene derivatives. J Phys Chem 95(26):10631–10643. https://doi.org/10.1021/j100179a026
Kaatz P, Donley EA, Shelton DP (1998) A comparison of molecular hyperpolarizabilities from gas and liquid phase measurements. J Chem Phys 108(3):849–856. https://doi.org/10.1063/1.475448
Stähelin M, Burland D, Rice J (1992) Solvent dependence of the second order hyperpolarizability in p-nitroaniline. Chem Phys Lett 191(3–4):245–250. https://doi.org/10.1016/0009-2614(92)85295-L
Adant C, Dupuis M, Bredas J (1995) Ab initio study of the nonlinear optical properties of urea: electron correlation and dispersion effects. Int J Quant Chem 56(S29):497–507. https://doi.org/10.1002/qua.560560853
Taha M, Ismail NH, Lalani S, Fatmi MQ, Siddiqui S, Khan KM, Imran S, Choudhary MI (2015) Synthesis of novel inhibitors of α-glucosidase based on the benzothiazole skeleton containing benzohydrazide moiety and their molecular docking studies. Eur J Med Chem 92:387–400. https://doi.org/10.1016/j.ejmech.2015.01.009
Taha M, Ismail NH, Baharudin MS, Lalani S, Mehboob S, Khan KM, Siddiqui S, Rahim F, Choudhary MI (2015) Synthesis crystal structure of 2-methoxybenzoylhydrazones and evaluation of their α-glucosidase and urease inhibition potential. Med Chem Res 24(3):1310–1324. https://doi.org/10.1007/s00044-014-1213-8
Zheng J-W, Ma L (2016) Metal complexes of anthranilic acid derivatives: a new class of non-competitive α-glucosidase inhibitors. Chin Chem Lett 27(5):627–630. https://doi.org/10.1016/j.cclet.2016.01.052
Acknowledgements
This work was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK) (Project Number: MFAG-117F235) and the Scientific Research Projects Unit of Sakarya University (Project Number: 2018-1-6-67).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no financial and non-financial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Avcı, D., Altürk, S., Sönmez, F. et al. Novel metal complexes containing 6-methylpyridine-2-carboxylic acid as potent α-glucosidase inhibitor: synthesis, crystal structures, DFT calculations, and molecular docking. Mol Divers 25, 171–189 (2021). https://doi.org/10.1007/s11030-020-10037-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10037-x