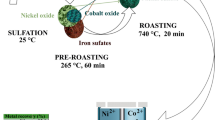
A characteristic feature of serpentine oxidized nickel ores (ONO) of the Serov deposit (≈ 1 wt.% Ni) is the increased content of iron and magnesium. The main nickel-containing phases are lizardite, antigorite, clinochlore and iron oxides with the nickel content varying from 1.6 to 9.2 wt.%.
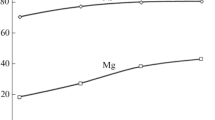
Heap (percolation) leaching with aqueous H2SO4 solutions was used for processing. During a pilot study, 89% of nickel, 99% of cobalt, and 78.4% of iron were extracted from the oxidized nickel ores during 440 days of leaching at an acid consumption rate of 475.1 kg per ton of ore. The high iron content of the product solutions did not allow for high-quality extraction of nickel and cobalt. The dependences of the nickel, cobalt, and iron extraction on the irrigation pauses, initial size of the ore fragments, and specific consumption of the leaching solution were studied. A solution was found on how to reduce the sulfuric acid consumption and considerably decrease the iron content of the product solutions by increasing a pause between irrigations at the initial stage of or leaching and redirecting the leaching solutions to treat fresh ore. In this case, iron is hydrolyzed in the form of insoluble hydroxides within the ore body. The optimal process conditions of heap leaching of such ores are as follows: initial size of ore fragments – 50 mm; concentration of H2SO4 solutions – decreasing from 20 to 5 g/dm3 during the leaching process; irrigation density – 140 dm3 per ton of ore; combination of the irrigation pauses from 4 days during the initial stages to 1 day during the subsequent stages. Given the average rate of H2SO4 consumption of 221 kg per 1 ton of ore and leaching duration of 408 days, these conditions make it possible to extract up to 62% of nickel and 66.7% of cobalt, as well as significantly reduce the percentage of iron leached from the ore to obtain iron-free product solutions suitable for extraction of nickel and cobalt.




Similar content being viewed by others
References
I. D. Reznik, G. P. Yermakov, and Ya. M. Shneerson, Nickel: in 3 Vol., Vol. 2, Oxidized Nickel Ores. Characteristics of Ores. Pyrometallurgy and Hydrometallurgy of Oxidized Nickel Ores [in Russian], OOO “Nauka i Tekhnologii,” Moscow (2004).
A.P. Stavskii (editor), Mineral Raw Materials from Earth Depths to the Market: in 3 Vol., Vol. 2, Non-ferrous Metals. Aluminum, Copper, Nickel, Tin, Lead, Zinc [in Russian], Nauchnyi Mir, Moscow (2011).
Information and Technical Reference Book. Nickel and Cobalt Production [in Russian], NDT Bureau, Moscow (2016).
Information and Technical Reference Book. Nickel and Cobalt Production [in Russian], NDT Bureau, Moscow (2019).
O. B. Kolmachikhina, Combined Technology of Processing Oxidized Nickel Ores (Case Study: Serov Deposit), Dissert. Cand. Sci. (Eng.); May 16, 2002; Yekaterinburg (2018).
M. I. Kalashnikova, L. B. Tsymbulov, S. S. Naboichenko, and O. B. Kolmachikhina, “Promising directions of processing oxidized nickel ores as applied to the ores from Ural deposits,” Tsvetnye Metally, No. 8, 4–11 (2019).
S. V. Sergeeva, Development of Electrothermal Technology for the Production of Ferronickel from Ural Serpentine Ores, Dissert. Cand. Sci. (Eng.); May 16, 2002; Yekaterinburg (2017).
A. M. Klushnikov, Development of the Technology of Joint Pyrometallurgical Processing of Oxidized Nickel and Copper Sulfide Ores, Dissert. Cand. Sci. (Eng.); May 16, 2002; Yekaterinburg (2017).
A. M. Koganovskii, N. A. Klimenko, T. M. Levchenko, R. M. Marutovskii, and I. G. Roda, Waste Water Treatment and Use in Industrial Water Supply [in Russian], Khimiya, Moscow (1983).
K. M. Smirnov, T. V. Molchanova, A. V. Ananyev, and O. K. Krylova, “Technology of hydrometallurgical processing of nickelmagnesia ores of the Aganozersk deposit,” Metally, No. 4, 13-18 (2018).
D. B. Baskov, S. V. Plekhanov, S. L. Orlov, and G. A. Sereda, Method of Extracting Nickel and Cobalt from Silicate Nickel Ores, RF Patent No. 2161658, IPC C 22 B 23/00, Appl. No. 2000116618/02; Filing date: June 28, 2000; Publ. date: January 10, 2001; Bul. 1.
R. G. McDonald and B. I. Whittington, “Atmospheric acid leaching of nickel laterites review. Part I. Sulphuric acid technologies,” Hydrometallurgy, No. 91, 35–55 (2008); “Part II. Chloride and Bio-Technologies,” Hydrometallurgy, 56–69.
G. S. Grebnev, N. V. Savenya, M. N. Savenya, and S. A. Sukleta, Method of Extracting Nickel and Cobalt from Nickel-Cobalt Silicate Ores, RF Patent No. 2465449, IPC E 21 B 43/28, Appl. No. 2011103543/03; Filing date: February 01, 2011; Publ. date: October 27, 2012; Bul. 30.
I. I. Kalinichenko, V. V. Vaitner, A. S. Molodykh, and V. N. Shubin, Method for Processing Oxidized Nickel Ores, RF Patent No. 2532871, IPC C 22 B 23/00, Appl. No. 2013118820/02; Filing date: April 23, 2013; Publ. date: November 10, 2014; Bul. 31.
B. Ma, W. Yang, B. Yang, C. Wang, Y. Chen, and Y. Zhang, “Pilot-scale plant study on the innovative nitric acid pressure leaching technology for laterite ores,” Hydrometallurgy, No. 155, 88–94 (2015).
S. Kursunoglu and M. Kaya, “Atmospheric pressure acid leaching of Caldag lateritic nickel ore,” Intern. J. of Mineral Processing, 150, 1–8 (2016).
G. S. Simate, S. Ndlovu, and L. F. Walubita, “Fungal and chemolithotrophic leaching of nickel laterites – Challenges and opportunities,” Hydrometallurgy, 103, 150–157 (2010).
G. S. Simate, S. Ndlovu, and M. Gericke, “Bacterial leaching of nickel laterites using chemolithotrophic microorganisms: Process optimization using response surface methodology and central composite rotatable design,” Hydrometallurgy, 98, 241–246 (2009).
Ye. A. Kim, Development of the Process of Bioleaching Nickel Silicate Ores of an Ferromagnesian Type [in Russian], Abstract Dissert. Cand. Sci. (Eng.), MISiS, Moscow (2010).
R. Barbaroux, G. Mercier, J. F. Blais, J. L. Morel, and M. O. Simonnot, “A new method for obtaining nickel from the hyperaccumulator plant Alyssum murale,” Separation and Purification Technology, 83, 57-65 (2011).
Prof. S. S. Naboichenko (editor), Ural Nickel. Developmental Trends: Round Table Materials [in Russian], Dr. Sci. (Eng.), UrFU, Yekateriburg (2013).
A. Oxley, M. R. Smith, and O. Caceres, “Why heap leach nickel laterites?” Minerals Engineering, 88, 53–60 (2016).
P. Yu. Chuvashov, N. A. Vatolin, and B. D. Khalezov, “Optimal conditions of leaching nickel and cobalt from oxidized nickel ores of the Serov deposit,” Khimicheskaya Tekhnologiya, 13, No. 2, 72–75 (2012).
A. I. Kalabin, Mineral Production by Underground Leaching and Other Geotechnological Methods [in Russian], Atomizdat, Moscow (1981).
J. Li, D. Li, Z. Xu, C. Liao, Y. Liu, and B. Zhong, “Selective leaching of valuable metals from laterite nickel ore with ammonium chloride-hydrocloric acid solution,” J. of Cleaner Production, 179, 24–30 (2018).
S. H. Ahoranta, M. K. Peltola, A.-M. Lakaniemi, and J. A. Puhakka, “Enhancing the activity of iron-oxidizing bacteria: A case study with process liquors from heap bioleaching of complex sulfide ores,” Hydrometallurgy, 167, 163–172 (2017).
B. D. Khalezov, A. S. Gavrilov, A. G. Krasheninin, and Ye. A. Zelenin, Method of Processing Oxidized Nickel Ores, RF Patent No. 2618595, IPC C 22 B 23/00, Appl. No. 2016111038; Filing date: March 24, 2016; Publ. date: May 04, 2017; Bul. 13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 66, No. 5, pp. 84–91, May, 2022. Russian DOI https://doi.org/10.52351/00260827_2022_05_84.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gavrilov, A.S., Krasheninin, A.G., Petrova, S.A. et al. Extraction of Nickel from Oxidized Nickel Ores by Heap Leaching. Metallurgist 66, 593–604 (2022). https://doi.org/10.1007/s11015-022-01364-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-022-01364-5