Abstract
The pandemic of Serious Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) that produces corona virus disease (COVID-19) has challenged the entire mankind by rapidly spreading globally in 210 countries affecting over 25 million people and about 1 million deaths worldwide. It continues to spread, afflicting the health system globally. So far there is no remedy for the ailment and the available antiviral regimens have been unsatisfactory for the clinical outcomes and the mode of treatment has been mainly supportive for the prevention of COVID-19-induced morbidity and mortality. From the time immortal the traditional plant-based ethno-medicines have provided the leads for the treatment of infectious diseases. Phytopharmaceuticals have provided potential and less toxic antiviral drugs as compared to conventional modern therapeutics which are associated with severe toxicities. The ethnopharmacological knowledge about plants has provided food supplements and nutraceuticals as a promise for prevention and treatment of the current pandemic. In this review article, we have attempted to comprehend the information about the edible medicinal plant materials with potential antiviral activity specifically against RNA virus which additionally possess property to improve immunity along with external and internal respiration and exhibit anti-inflammatory properties for the prevention and treatment of the disease. This will open an arena for the development of novel nutraceutical herbal formulations as an alternative therapy that can be used for the prevention and treatment of COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
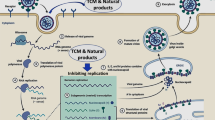
In Wuhan, China in December 2019, a newly emergent novel coronavirus SARS-CoV-2 was reported to cause severe acute respiratory tract infections, coronavirus disease 2019 (COVID-19) [1]. After the first case of corona reported from Wuhan, there has been an unprecedented outbreak of COVID-19. As of the last week of August 2020, over 25 million cases of this disease have been reported from 210 countries with 3.32% deaths [2, 3]. The United Nations called the pandemics of COVID-19 as the worst global humanitarian crisis since World War II. Countries all over the world are taking aggressive steps and adopting all possible preventive measures to combat the spread of this disease. The disease is associated with high mortality risk (2–8% in different countries), a very high transmission rate combined with the lack of WHO or FDA approved specific prophylactic vaccines or therapeutic protocols for the effective prevention, treatment or management of the disease. A typical viral disease mechanism involves the entry of virus into the host via specific receptor, followed by uncoating, transcription and genome synthesis finally forming viral assembly and releasing of multiple copies in the host. The antiviral drugs are designed to act on such varied targets (Fig. 1).
The current treatment appears to be mainly supportive in nature [4, 5]. From time immemorial, herbal medicine has provided remedies for majority of the diseases, for e.g., digitalis and reserpine for cardiac patients, artemisinin and quinine for malaria, vincristine and vinblastine for cancer. Some potential drug candidates including blockbuster antivirals like Remdesivir, Hydroxychloroquine, Lopinavir, Ritonavir, APN01 or Favipiravir are being tested for clinical trials across the globe. Still no therapy has been found to be effective or devoid of deleterious effects against COVID-19 as of now [6,7,8]. Kee** in view the shortcomings associated with available antiviral drugs therapies, i.e. viral resistance coupled with the problem of viral latency and conflicting efficacy in recurrent infection in immunocompromised patients, there is an increasing need for search of new compounds and therapy with antiviral activity that are highly efficacious and cost-effective for the management and control of viral infections. Moreover, the viral infections caused by Coronaviruses, Human immunodeficiency virus, Ebola virus, Nipah virus, Influenza virus, Enterovirus, Swine flu, Bird flu, Zika Virus, Hepatitis B and C, etc. have risen significantly and natural products play a vital role in the cure for some with no or less harmful effects [9, 10]. Novel bioactive phytomolecules bearing credible therapeutic potential against such viral diseases is the prime focus of the current medical research in order to gain an upper edge over such widespread infections and prevent the future ones [11, 12]. Identification of the antiviral mechanisms from these natural agents has shed light for further research targeting virus–host-specific interactions.
Indian subcontinent has been recognized as a treasure home for various plant species due to its varied agro-climatic zones and suitable topographical conditions and is placed among the list of top 12 mega diversity countries of the world. The Indian subcontinent is endowed with rich and diverse flora about which the ethnobotanical literature describes use as plant extracts, infusions and powders for diseases of infectious nature [13]. These medicinal herbs provide a wide approach in managing several diseases, including viral respiratory infections by modulating immune system and inflammatory responses. AYUSH system of medicine has provided a basic approach on prevention of infection through dietary modification, lifestyle management, remedies to boost immune system and preventive interventions based on the symptom [14]. Around 65–80% of the world population residing in develo** countries utilizes traditional herbs in their primary health treatment. Additionally, the interest in the study of herbs have aroused due to their phytoactive/phytotherapeutic agents which can be utilized in the form of nutraceuticals, which possess drug-like actions, and in some cases can be traced directly through the existing links between a local and biomedical use [15]. The lack of preventive and curative treatment of COVID-19 till date compels the researchers to look onto therapeutic alternatives that can be added in our daily diets in order to both prevent and cure such life-threatening infections and provide long-term protection. For centuries, numerous plants have been used in daily diets which serve as folk remedies by supporting the body system in one way or the other [16].
This review aims to bring focus on the detailed information about herbal flora with antiviral activities that can be explored for development of novel nutraceutical herbal formulations. Advancement in separation technologies, adoption of modern drug discovery and the development of vector-based strategies for antiviral screening purposes offers a promise for edible medicinal plants usage in daily diet, and may serve as an alternative therapy for treatment of this pandemic and prevention of another one. The article also aimed to merge the ethnopharmacological knowledge with the modern technologies to devise drug targets for the SARS-COV-2 virus and identification of potential candidates from natural sources which may offer some preventive or even therapeutic value.
Pathogenesis of COVID-19 and strategy for using edible plants
For a better insight on how nutraceuticals or phytomolecules can effectively work against novel coronaviruses, it is imperative to understand the structural characteristics and culpable targets and receptors associated with it. Moreover, understanding the mechanism of action of conventional antivirals and liable targets for drug designing may be useful for development of therapy regimen for COVID-19 from natural sources. SARS-CoV-2 like other HCoVs is positive-sense single-stranded RNA viruses with two groups of protein forming its characteristic markers: structural protein, such as Spike (S), Nucleocapsid (N), Matrix (M), Envelope (E); and non-structural proteins such as nsp12-RNA-dependent RNA polymerase (RdRp), Nsp3- Papain-like proteinases, Nsp5-3C-like main protease and nsP13 SARS-CoV helicase [17, 18]. Primarily, nsP13 helicase, 3CL protease, nsp12-RNA-dependent RNA polymerase (RdRp) become primary target for drug development. Apart from these proteins, the viral spike glycoprotein (S) initial attachment and internalization within host cells ACE-2 receptor can also be targeted to prevent viral entry into newer host cells [19]. SARS-CoV-2 recognizes human angiotensin-converting-enzyme-2 (ACE-2), thereby proving its essentiality for host cell entry by invasion of alveolar epithelial cells, subsequent viral replication and primary host lung cells infection as ACE-2 is highly expressed in the heart, lungs, intestine, kidney and blood vessels [20]. The expression of ACE-2 is substantially increased in patients with diabetes and hypertension and the connecting link to this associated comorbidity has been the angiotensin-converting-enzyme-2 (ACE-2) receptor as it is the site of virus multiplication, and thus a strategy has been devised to develop antiviral newer drugs considering the ACE-2 as an attractive target [21]. Various plants produce phytomolecules that can be utilized in targeting these viral targets and so has been done previously in case of other viral diseases like SARS, HIV, HCV, etc. [22,23,24].
At the molecular level, the virus SARS-CoV-2 binds to the angiotensin-converting-enzyme-2 (ACE-2) present in the lungs of the human host. Binding of the virus to the host cells through its trimeric spike glycoprotein makes this protein a key target for potential therapies and diagnostics. It was reported that in SARS-CoV-2, the S2 subunit in each spike monomer contains a fusion peptide, a transmembrane domain, and cytoplasmic domain which is highly conserved and could be a possible target for antiviral (anti-S2) compounds [25]. There occurs multiplication of the viruses that induces cellular responses. There occurs infiltration of huge number of inflammatory cells which comprise innate immune cells and adaptive immune cells [26, 34]. Herbal preparations that possess immunomodulatory activity may serve as prophylactic treatment, if added in daily diet, for prevention of infection acquisition during this spell of critical community level spread and help contain the disease in community as well as help faster healing post infection. Considering the above strategies for treatment, management and prevention of COVID- 19, a search for potential plants with above properties can help to devise natural plant-derived antiviral agents against the pandemic disease.
Edible plants exhibiting antiviral property against RNA viruses: initial signals for COVID-19
The secondary metabolites obtained from herbal drugs can also be utilized as nutraceuticals and can become a lead compound in the treatment therapy [35]. Studies have also shown promising results of nutraceuticals and phytomolecules in various pathological complications such as diabetes, atherosclerosis, cardiovascular diseases (CVDs), cancer and neurological disorders [14]. Since ages, herbs of Indian origin have been implemented in treatment and as preventive strategies for several diseases that include respiratory viral infections as the benefit of usage of these herbs against viral respiratory infections lies in immune stimulation and inflammation modulating effects. The AYUSH systems of medicine also promotes prevention of COVID-19 through lifestyle modification as well as dietary management and prophylactic interventions for improving the immunity [31]. All these have led to a revival of interest in herbal medicines, novel nutraceuticals and herbal formulations with antiviral potency based on any of the potential plants. Looking into the results of previously deciphered phytochemical-directed researches, a wide variety of phytomolecules present in Indian forest biodiversity can point towards their capability to be manipulated into devising antiviral drugs for SARS-CoV-2. Table 1 provides detailed information on edible plants used as food or nutraceutical showing antiviral activity against RNA viruses, their potential to be explored against COVID-19 on the basis of antiviral activity against various RNA viruses, their active phytoconstituents bearing potential anti-coronaviral activity and mechanism of action.
Plants preventing entry of virus in the host
It has been reported that flavonoids can bind to the functional domains of the SARS-CoV-2S protein, a viral surface glycoprotein required for initial attachment and internalization within host cells. Emodin from plants of family polygonaceae can block the interaction with the SARS-coronavirus spike protein by inhibiting the 3a ion channel of SARS-CoV and HCoV-OC43 [36]. Lectins, the natural proteins, also target the sugar moieties of a SARS-CoV spike protein. In time-of-addition assay conducted to understand mechanism of antiviral action, glucose-, galactose-, N-acetyl glucosamine- and N-acetyl galactosamine binding lectins and most importantly mannose binding lectin indicated their interference with virus attachment to spike protein making them early entry inhibitors. Lectins also carry prophylactic potentials as it agglutinates viral particles by binding to it, thereby not allowing it to bind to human cell receptors and complete its pathogenic cycle [37]. As SARS-CoV-2 also uses host receptor ACE-2 for the cellular entrance similar to SARS-CoV [38], medicinal herbs with the capacity to target ACE-2 therefore holds a promising effect in the prevention and infection of SARS-CoV-2. Various edible medicinal plants, including Cynara scolymus [39], Cassia occidentalis [40] and Punica granatum [41], have shown ACE inhibitory effects, and the same can be explored for inhibition of ACE2 also.
Plants inhibiting viral replication
Studies on edible plants, such as Glycyrrhiza glabra [42], Allium sativum [43], showed the inhibition of viral replication of SARS-CoV that can be further utilized as leads against SARS-CoV-2, due to similar homology between SARS-CoV and SARS-CoV-2 [44]. Edible antiviral plants like Aloe vera [45], Gingko [46], Olea europaea [47], Cicer arietinum [48], Nigella sativa [49], Agrimonia Pilosa [50], Commelina communis [51], Mangifera indica [52], Syzygium cumini [53] that showed effects against influenza virus can be studied rigorously to investigate any relatable target between SARS-CoV-2 and influenza virus.
Myricetin and scutellarein can act as novel chemical inhibitors of the SARS coronavirus helicase, nsP13 [54]. Flavonoids isolated from medicinal plants have been reported to show antiviral activity. Quercetin, epigallocatechin gallate and gallocatechin gallate showed inhibitory activity against 3CLpro of SARS-CoV [55]. Plants showing inhibitory effects on HIV proteases, such as Eugenia jambolana, Areca nut [56], can be investigated for their effects on SARS-CoV-2. Similarly, plants like Ocimum sanctum [57], Phaseolus vulgaris [58], Phyllanthus emblica [59] having HIV reverse transcriptase activity can also be studied against SARS-CoV-2. Plants like Solanum nigrum [60] have been known to target the reverse transcriptase activity of HIV and can be studied for activity against SARS-CoV-2 as well; betulinic acid, savinin and some plant-based phenolic compounds are competitive inhibitors of SARS-CoV 3CL protease [61]. Azadirachta indica inhibits viral replication in Group B Coxsackieviruses virus and can be investigated for their possible effects against SARS-CoV-2 [62]. Another herb Aegle marmelos inhibited viral replication in human coxsackieviruses B1-B6 infection and can be used in the study against SARS-CoV-2 [62]. Another potential target that can be utilized for the inhibition of CoV replication is proteases [63]. Trachyspermum ammi [64] and Solanum nigrum [65] inhibited viral protease enzymes in hepatitis C virus (HCV) infection. Acalypha indica showed selective anti-VSV activity by protein interaction [64], and Ocimum sanctum also inhibited HIV protease enzyme [57]; therefore these plants can be studied against SARS-CoV-2 as they may target protease enzymes.
Plants inhibiting viral envelop formation
Sambucus ebulus has been known to inhibit the activity of enveloped viruses and can also be used to target this virus. Though the detailed mechanism remains unclear, Sambucus ebulus is indicated to inhibit the entry of enveloped viruses owing to the presence of lectins that block viral entry. Phenolic compounds like quertin 3–0-glucoside and isorhamnetin present in the plant have previously demonstrated the prophylactic potential against Ebola virus. The flavonoids, diosgenin and yomogenin of Sambucus species also showed viral entry inhibition against Hepatitis C viruses [61].
Antiviral plants with unknown mechanism
A study on Abutilon indicum, Gymnema sylvestre, Leucas aspera showed anti-mouse coronaviral activity which is a surrogate of human SARS virus but its mechanism of action is still unexplored and requires more research in this area [66]. Leucas aspera has been shown to have anti-MCV and anti-HSV activities, Abutilon indicum extract was found active against influenza virus and Sindbis virus which is a surrogate to Hepatitis B virus. Gymnemic acid from Gymnema sylvestre has virucidal activity against Asian influenza virus, whereas Artemisia annua showed inhibitory effects against SARS-CoV and likely against SARS-CoV-2 but their mechanism of action is still unknown [47, 68]. On the other hand, Chebulagic acid and chebulinic acid from Terminalia chebula have shown efficacy to inhibit virus attachment and penetration comparable to Acyclovir as well as implement neuraminidase-mediated viral release similar to the antiviral drug oseltamivir [ WHO (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization, Kusumoto IT, Nakabayashi T, Kida H, Miyashiro H, Hattori M, Namba T, Shimotohno K (1995) Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Phytother Res 9(3):180–184. https://doi.org/10.1002/ptr.2650090305 University JH (2020) COVID-19 Dash board by the Center for Systems Science and Engineering (CSSE) at JHU. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed 13 May 2020 Dayer MR, Taleb-Gassabi S, Dayer MS (2017) Lopinavir; a potent drug against coronavirus infection: insight from molecular docking study. Arch Clin Infect Dis. https://doi.org/10.5812/archcid.13823 Omolo C, Soni N, Fasiku V, Mackraj I, Govender T (2020) Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2020.173348 Wang Y, Zhang D, Du G, Du R, Zhao J, ** Y, Fu S, Gao L, Cheng Z, Lu Q (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395(10236):1569–1578. https://doi.org/10.1016/S0140-6736(20)31022-9 Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16(3):155–166. https://doi.org/10.1038/s41584-020-0372-x Jorge A, Ung C, Young LH, Melles RB, Choi HK (2018) Hydroxychloroquine retinopathy—implications of research advances for rheumatology care. Nat Rev Rheumatol 14(12):693–703. https://doi.org/10.1038/s41584-018-0111-8 Vijayan P, Raghu C, Ashok G, Dhanaraj S, Suresh B (2004) Antiviral activity of medicinal plants of Nilgiris. Indian J Med Res 120:24–29 Lin L-T, Hsu W-C, Lin C-C (2014) Antiviral natural products and herbal medicines. J Tradit Complement Med 4(1):24–35. https://doi.org/10.4103/2225-4110.124335 Chung T, Kim J, Kim M, Choi S, Kim S, Chung J, Lee I, Kim SH, Hahn K, Lee I (1995) Investigation of Korean plant extracts for potential phytotherapeutic agents against B-virus hepatitis. Phytother Res 9(6):429–434. https://doi.org/10.1002/ptr.2650090609 Akram M, Tahir IM, Shah SMA, Mahmood Z, Altaf A, Ahmad K, Munir N, Daniyal M, Nasir S, Mehboob H (2018) Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother Res 32(5):811–822. https://doi.org/10.1002/ptr.6024 Vijayalatha S (2004) An Ornamental garden with medicinal plants an indirect approach for conservation of medicinal plants. J Indian J Arecanut Spices Med Plants 6(3):98–107. https://doi.org/10.1002/mnfr.201601066 Zhao J (2007) Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: a perspective on plant biotechnology application. Recent Pat Biotechnol 1(1):75–97. https://doi.org/10.2174/187220807779813893 Patra JK, Das G, Kumar S, Thatoi H (2019) Ethnopharmacology and Biodiversity of Medicinal Plants. CRC Press, pp 470 Vanden Berghe D, Vlietinck A, Van Hoof L (1986) Plant products as potential antiviral agents. Bull Inst Pasteur 84(2):101–147. https://doi.org/10.3923/ajava.2011.1125.1152 Elfiky AA (2020) Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci 248:117477. https://doi.org/10.1016/j.lfs.2020.117477 Barnard DL, Kumaki Y (2011) Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol 6(5):615–631. https://doi.org/10.2217/fvl.11.33 Belouzard S, Millet JK, Licitra BN, Whittaker GR (2012) Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 4(6):1011–1033. https://doi.org/10.3390/v4061011 Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor recognition by the novel Coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J Virol 94(7):e00127-e1120. https://doi.org/10.1128/JVI.00127-20 Majeed J, Ajmera P, Goyal RK (2020) Delineating clinical characteristics and comorbidities among 206 COVID-19deceasedpatients in India: Emerging significance of renin angiotensin system derangement. Diabetes Res Clin Pract. https://doi.org/10.1016/j.diabres.2020.108349 Scotti N, Buonaguro L, Tornesello ML, Cardi T, Buonaguro FM (2010) Plant-based anti-HIV-1 strategies: vaccine molecules and antiviral approaches. Expert Rev Vaccines 9(8):925–936. https://doi.org/10.1586/erv.10.79 Ho T-Y, Wu S-L, Chen J-C, Li C-C, Hsiang C-Y (2007) Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res 74(2):92–101. https://doi.org/10.1016/j.antiviral.2006.04.014 Reddy BU, Mullick R, Kumar A, Sudha G, Srinivasan N, Das S (2014) Small molecule inhibitors of HCV replication from pomegranate. Sci Rep 4:5411. https://doi.org/10.1038/srep05411 Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020) Features, evaluation and treatment coronavirus (COVID-19). In: Statpearls [internet]. StatPearls Publishing, Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8(4):420–422. https://doi.org/10.1016/S2213-2600(20)30076-X Tian S, Hu W, Niu L, Liu H, Xu H, **ao S-Y (2020) Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 15(5):700–704. https://doi.org/10.1016/j.jtho.2020.02.010 Tomar B, Anders H-J, Desai J, Mulay SR (2020) Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells 9(6):1383. https://doi.org/10.3390/cells9061383 Small BA, Dressel SA, Lawrence CW, Drake DR III, Stoler MH, Enelow RI, Braciale TJ (2001) CD8+ T cell–mediated injury in vivo progresses in the absence of effector T cells. J Exp Med 194(12):1835–1846. https://doi.org/10.1084/jem.194.12.1835 Channappanavar R, Perlman S (2017) Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In: Seminars in immunopathology vol 39. Springer Berlin Heidelberg., pp 529–539. doi:https://doi.org/10.1007/s00281-017-0629-x Rizzo P, Dalla Sega FV, Fortini F, Marracino L, Rapezzi C, Ferrari R (2020) COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol 115(3):31. https://doi.org/10.1007/s00395-020-0791-5 Ye Q, Wang B, Mao J (2020) The pathogenesis and treatment of the cytokine storm in COVID-19. J Infect 8(6):607–613. https://doi.org/10.1016/j.**f.2020.03.037 Letko M, Marzi A, Munster V (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5:562–569. https://doi.org/10.1038/s41564-020-0688-y Prompetchara E, Ketloy C, Palaga T (2020) Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 38(1):1–9. https://doi.org/10.12932/AP-200220-0772 Heinrich M, Gibbons S (2001) Ethnopharmacology in drug discovery: an analysis of its role and potential contribution. J Pharm Pharmacol 53(4):425–432. https://doi.org/10.1211/0022357011775712 Schwarz S, Wang K, Yu W, Sun B, Schwarz W (2011) Emodin inhibits current through SARS-associated coronavirus 3a protein. Antiviral Res 90(1):64–69. https://doi.org/10.1016/j.antiviral.2011.02.008 Keyaerts E, Vijgen L, Pannecouque C, Van Damme E, Peumans W, Egberink H, Balzarini J, Van Ranst M (2007) Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res 75(3):179–187 Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273. https://doi.org/10.1038/s41586-020-2012-7 Elsebai MF, Mocan A, Atanasov AG (2016) Cynaropicrin: a comprehensive research review and therapeutic potential as an anti-hepatitis C virus agent. Front Pharmacol 7:472. https://doi.org/10.3389/fphar.2016.00472 Lunavath V, Estari (2012) Inhibition of Human Immunodeficiency Virus (HIV-1) Reverse Transcriptase by Casia occidentalis (L) Plant Extract. Int J Eng Res 3 (7) Khan MY, Kumar V (2018) Mechanism & inhibition kinetics of bioassay-guided fractions of Indian medicinal plants and foods as ACE inhibitors. J Tradit Complement Med 9(1):73–84. https://doi.org/10.1016/j.jtcme.2018.02.001 Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H (2003) Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet 361(9374):2045–2046. https://doi.org/10.1016/s0140-6736(03)13615-x Weber ND, Andersen DO, North JA, Murray BK, Lawson LD, Hughes BG (1992) In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med 58(5):417–423. https://doi.org/10.1055/s-2006-961504 Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J (2020) Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 27(3):325–328. https://doi.org/10.1016/j.chom.2020.02.001 Sydiskis R, Owen D, Lohr J, Rosler K, Blomster R (1991) Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother 35(12):2463–2466. https://doi.org/10.1128/aac.35.12.2463 Miki K, Nagai T, Suzuki K, Tsujimura R, Koyama K, Kinoshita K, Furuhata K, Yamada H, Takahashi K (2007) Anti-influenza virus activity of biflavonoids. Bioorg Med Chem Lett 17(3):772–775. https://doi.org/10.1016/j.bmcl.2006.10.075 Micol V, Caturla N, Pérez-Fons L, Más V, Pérez L, Estepa A (2005) The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV). Antiviral Res 66(2–3):129–136. https://doi.org/10.1016/j.antiviral.2005.02.005 Kan A, Özçelİk B, Kartal M (2009) In vitro antiviral activities under cytotoxic doses against herpes simples type-1 and parainfluensa-3 viruses of Cicer arietinum L. (Chickpea). Afr J Pharm Pharmacol 3(12):627–631 Umar S, Munir MT, Subhan S, Azam T, Nisa Q (2016) Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys. J Saudi Soc Agric Sci Shin WJ, Lee KH, Park MH, Seong BL (2010) Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol Immunol 54(1):11–19. https://doi.org/10.1111/j.1348-0421.2009.00173.x Zhang GB, Bing FH, Liu J, Li Z, Liao YF, Li J, Dong CY (2010) Effect of total alkaloids from Commelina communis L on lung damage by influenza virus infection. Microbiol Immunol 54(12):754–757. https://doi.org/10.1111/j.1348-0421.2010.00277.x Abdel-Mageed WM, Bayoumi SAH, Chen C, Vavricka CJ, Li L, Malik A, Dai H, Song F, Wang L, Zhang J, Gao GF, Lv Y, Liu L, Liu X, Sayed HM, Zhang L (2014) Benzophenone C-glucosides and gallotannins from mango tree stem bark with broad-spectrum anti-viral activity. Bioorg Med Chem 22(7):2236–2243. https://doi.org/10.1016/j.bmc.2014.02.014 Sood R, Swarup D, Bhatia S, Kulkarni D, Dey S, Saini M, Dubey S (2012) Antiviral activity of crude extracts of Eugenia jambolana Lam. against highlypathogenic avian influenza (H5N1) virus. Indian J Exp Biol 50:179–186 Yu M-S, Lee J, Lee JM, Kim Y, Chin Y-W, Jee J-G, Keum Y-S, Jeong Y-J (2012) Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg Med Chem Lett 22(12):4049–4054. https://doi.org/10.1016/j.bmcl.2012.04.081 Musarra-Pizzo M, Ginestra G, Smeriglio A, Pennisi R, Sciortino MT, Mandalari G (2019) The antimicrobial and antiviral activity of polyphenols from Almond (Prunus dulcis L.) skin. Nutrients 11(10):2355. https://doi.org/10.3390/nu11102355 Inokuchi J, Okabe H, Yamauchi T, Nagamatsu A, Nonaka G, Nishioka I (1986) Antihypertensive substance in seeds of Areca catechu L. Life Sci 38(15):1375–1382. https://doi.org/10.1016/0024-3205(86)90470-4 Rege A, Chowdhary AS (2014) Evaluation of Ocimum sanctum and Tinospora cordifolia as probable HIV protease inhibitors. Int J of Pharm Sci Rev Res 25:315–318 Ye XY, Ng TB, Tsang PW, Wang J (2001) Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J Protein Chem 20(5):367–375 Hossan MS, Fatima A, Rahmatullah M, Khoo T, Nissapatorn V, Galochkina A, Slita A, Shtro A, Nikolaeva Y, Zarubaev V, Wiart C (2018) Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch Virol 163(8):2121–2131. https://doi.org/10.1007/s00705-018-3842-6 Yu Y-B (2004) The extracts of Solanum nigrum L for inhibitory effects on HIV-1 and its essential enzymes. Korean J Orient Med Prescr 10(1):119–126 Chowdhury M, Shahid M, Kashem M (2020) Scope of natural plant extract to deactivate COVID-19. [Preprint]. https://doi.org/10.21203/rs.3.rs-19240/v1 Badam L, Bedekar SS, Sonawane KB, Joshi SP (2002) In vitro antiviral activity of bael (Aegle marmelos Corr) upon human coxsackieviruses B1–B6. J Commun Dis 34(2):88–99 Kilianski A, Mielech AM, Deng X, Baker SC (2013) Assessing activity and inhibition of Middle East respiratory syndrome coronavirus papain-like and 3C-like proteases using luciferase-based biosensors. J Virol 87(21):11955–11962. https://doi.org/10.1128/JVI.02105-13 Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K (2000) Inhibitory effects of sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res 14(7):510–516. https://doi.org/10.1002/1099-1573(200011)14:7%3c510::aid-ptr646%3e3.0.co;2-b Javed T, Ashfaq UA, Riaz S, Rehman S, Riazuddin S (2011) In-vitro antiviral activity of Solanum nigrum against hepatitis C virus. Virol J 8:26. https://doi.org/10.1186/1743-422X-8-26 Vimalanathan S, Ignacimuthu S, Hudson J (2009) Medicinal plants of Tamil Nadu(Southern India) are a rich source of antiviral activities. Pharm Biol 47:422–429. https://doi.org/10.1080/13880200902800196 Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, Zhang X, Hua SN, Yu J, **ao PG, Li RS, Tan X (2005) Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 67(1):18–23. https://doi.org/10.1016/j.antiviral.2005.02.007 San Chang J, Wang KC, Yeh CF, Shieh DE, Chiang LC (2013) Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol 145(1):146–151. https://doi.org/10.1016/j.jep.2012.10.043 **e F (2016) Broad-spectrum antiviral effect of chebulagic acid and punicalagin on respiratory syncytial virus infection in a BALB/c model. Int J Clin Exp Pathol 9(2):611–619. https://doi.org/10.1186/1471-2180-13-187 Yadav V, Mishra K, Singh D, Mehrotra S, Singh V (2005) Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol 27(3):485–497. https://doi.org/10.1080/08923970500242244 Im SA, Lee YR, Lee YH, Lee MK, Park YI, Lee S, Kim K, Lee CK (2010) In vivo evidence of the immunomodulatory activity of orally administered Aloe vera gel. Arch Pharm Res 33(3):451–456. https://doi.org/10.1007/s12272-010-0315-1 Badam L, Joshi S, Bedekar S (1999) 'In vitro’antiviral activity of neem (Azadirachta indica. A. Juss) leaf extract against group B Coxsackieviruses. J Commun Dis 31(2):79–90 Lu H (2020) Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 14(1):69–71. https://doi.org/10.5582/bst.2020.01020 Grover A, Agrawal V, Shandilya A, Bisaria VS, Sundar D Non-nucleosidic inhibition of Herpes simplex virus DNA polymerase: mechanistic insights into the anti-herpetic mode of action of herbal drug withaferin A. In: BMC bioinformatics, 2011. vol 13. BioMed Central, p S22. doi:https://doi.org/10.1186/1471-2105-12-S13-S22 Matsumoto M, Mukai T, Furukawa S, Ohori H (2005) Inhibitory effects of epigallocatechin gallate on the propagation of bovine coronavirus in Madin-Darby bovine kidney cells. Anim Sci J 76(5):507–512. https://doi.org/10.1111/j.1740-0929.2005.00297.x Coppin JP, Xu Y, Chen H, Pan M-H, Ho C-T, Juliani R, Simon JE, Wu Q (2013) Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J Funct Foods 5(4):1892–1899. https://doi.org/10.1016/j.jff.2013.09.010 Lii CK, Chen HW, Yun WT, Liu KL (2009) Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviata ser) fruit extracts on inflammatory responses in RAW 2647 macrophages. J Ethnopharmacol 122(2):227–233. https://doi.org/10.1016/j.jep.2009.01.028 Chan YS, Wong JH, Fang EF, Pan W, Ng TB (2013) A hemagglutinin from northeast red beans with immunomodulatory activity and anti-proliferative and apoptosis-inducing activities toward tumor cells. Protein Pept Lett 20(10):1159–1169. https://doi.org/10.2174/0929866511320100011 Ding X, Zhu F, Gao S (2012) Purification, antitumour and immunomodulatory activity of water-extractable and alkali-extractable polysaccharides from Solanum nigrum L. Food Chem 131(2):677–684. https://doi.org/10.1016/j.foodchem.2011.09.060 Chen Y, Guo JJ, Healy DP, Zhan S (2007) Effect of integrated traditional Chinese medicine and western medicine on the treatment of severe acute respiratory syndrome: a meta-analysis. Pharmacy Practice 5(1):1–9 Hsu C-H, Hwang K-C, Chao C-L, Chang SG, Ker C-C, Chien L-C, Ho M-S, Lin J-G, Chen Y-M, Chou P (2006) The lesson of supplementary treatment with Chinese medicine on severe laboratory-confirmed SARS patients. Am J Chin Med 34(06):927–935. https://doi.org/10.1142/S0192415X06004405 Hsu C-H, Hwang K-C, Chao C-L, Chang SG, Ho M-S, Chou P (2006) Can herbal medicine assist against avian flu? Learning from the experience of using supplementary treatment with Chinese medicine on SARS or SARS-like infectious disease in 2003. J Altern Complement Med 12(6):505–506. https://doi.org/10.1089/acm.2006.12.505 Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105932 New Coronavirus Pneumonia Dignosis and Treatment Program (2020) Office of the National Health and Health Commission Office of the State Administration of Traditional. http://www.nhc.gov.cn/xcs/zhengcwj/202002/8334a8326dd94d329df351d7da8aefc2.shtml. Accessed 3 May 2020 Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L, Li S-B, Wang H-Y, Zhang S, Gao H-N (2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. https://doi.org/10.1136/bmj.m606 Saini A, Gahlawat DK, Chauhan C, Gulia SK, Ganie SA, Archita YSS (2015) Ethnomedicinal uses and phytochemistry of Abutilon indicum (Linn.) Sweet: an overview. J Pharmacogn Phytochem 3(5):66–72 Reddy KN, Pattanaik C, Reddy CS, Raju VS (2007) Traditional knowledge on wild food plants in Andhra Pradesh. Indian J Tradit Know 6(1):223–229 Muzammil S, Manikandan M, Jafar A, Sakthivel P, Geetha S, Malarkodi R (2014) Anti-inflammatory studies on Acalypha indica L. leaves by membrane stabilization. Indian J Nat Prod Resour 5(2):195–197 Amalraj A, Pius A (2015) Bioavailability of calcium and its absorption inhibitors in raw and cooked green leafy vegetables commonly consumed in India: an in vitro study. Food Chem 170:430–436. https://doi.org/10.1016/j.foodchem.2014.08.031 Ali A, Mackeen M, Ei-Sharkawyl S, Hamidi J, Ismaili NOR, Ahmad H, Lajisi F (1996) Antiviral and cytotoxic activities of some plants used in Malaysian indigenous medicine. Pertanika J Trop Agric Sci 19:129–136 Baliga MS, Mane PP, Joseph N, Jimmy R, Watson RR, Preedy VR (2013) Chapter 20 - Review on the Protective Effects of the Indigenous Indian Medicinal Plant, Bael (Aegle marmelos Correa), in Gastrointestinal Disorders. In: Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease. Academic Press, San Diego, pp 313–324. doi:https://doi.org/https://doi.org/10.1016/B978-0-12-397154-8.00036-1 Kunkel G (1984) Plants for human consumption. Koeltz Scientific Books, Koenigstein Touloupakis E, Ghanotakis D (2010) Nutraceutical use of garlic sulfur-containing compounds. Adv Exp Med Biol 698:110–121. https://doi.org/10.1007/978-1-4419-7347-4_9 Tiwari R (2011) Garlic as food, spice and Medicine: as prospective. J Pharm Res 4:1857–1860 Ahlawat KS, Khatkar BS (2011) Processing, food applications and safety of aloe vera products: a review. J Food Sci Technol 48(5):525–533. https://doi.org/10.1007/s13197-011-0229-z Sun Z, Yu C, Wang W, Yu G, Zhang T, Zhang L, Zhang J, Wei K Aloe Polysaccharides Inhibit Influenza A Virus Infection-A Promising Natural Anti-flu Drug. Front Microbiol 9 (2338). doi:https://doi.org/10.3389/fmicb.2018.02338 Khan S, Mehmood MH, Ali ANA, Ahmed FS, Dar A, Gilani A-H (2011) Studies on anti-inflammatory and analgesic activities of betel nut in rodents. J Ethnopharmacol 135(3):654–661. https://doi.org/10.1016/j.jep.2011.03.064 Fazal F, Mane PP, Rai MP, Thilakchand KR, Bhat HP, Kamble PS, Palatty PL, Baliga MS (2014) The phytochemistry, traditional uses and pharmacology of Piper betel linn (Betel Leaf): a pan-asiatic medicinal plant. Chin J Integr Med. https://doi.org/10.1007/s11655-013-1334-1 Melillo de Magalhães P, Dupont I, Hendrickx A, Joly A, Raas T, Dessy S, Sergent T, Schneider Y (2012) Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem 134(2):864–871. https://doi.org/10.1016/j.foodchem.2012.02.195 Agize M (2016) Ethnobotany of spice and condiment plants and the associated indigenous knowledge on management, utilization and conservation of them in and around home gardens in Loma and Gena Bosa Districts ( Weredas ) of Dawuro Zone, Southern Ethiopia. Int J Agric Innov Res 4(3):426–442 Keservani RK, Kesharwani RK, Sharma AK, Vyas N, Chadokar A (2010) Nutritional supplements: an overview. J Curr Pharm Rev Res 1(1):59–75 Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T (2002) Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antiviral Res 53(1):19–34. https://doi.org/10.1016/s0166-3542(01)00189-9 Harbowy ME, Balentine DA, Davies AP, Cai Y (1997) Tea Chemistry. Crit Rev Plant Sci 16(5):415–480. https://doi.org/10.1080/07352689709701956 Lee HJ, Lee YN, Youn HN, Lee DH, Kwak JH, Seong BL, Lee JB, Park SY, Choi IS, Song CS (2012) Anti-influenza virus activity of green tea by-products in vitro and efficacy against influenza virus infection in chickens. Poult Sci 91(1):66–73. https://doi.org/10.3382/ps.2011-01645 Panigrahi G, Yadav A, Mandal P, Tripathi A (2016) Immunomodulatory potential of Rhein, an anthraquinone moiety of Cassia occidentalis seeds. Toxicol Lett. https://doi.org/10.1016/j.toxlet.2016.01.006 Manikandaselvi S, Vadivel V, Brindha P (2016) Studies on physicochemical and nutritional properties of aerial parts of Cassia occidentalis L. J Food Drug Anal 24(3):508–515 Ibrikci H, Knewtson SJB, Grusak MA (2003) Chickpea leaves as a vegetable green for humans: evaluation of mineral composition. J Sci Food Agric 83(9):945–950. https://doi.org/10.1002/jsfa.1427 Zhang GB, Tian LQ, Li YM, Liao YF, Li J, Bing FH (2013) Protective effect of homonojirimycin from Commelina communis (dayflower) on influenza virus infection in mice. Phytomedicine 20(11):964–958. https://doi.org/10.1016/j.phymed.2013.04.009 Britta MO, Ho Thi T, Hoang Nghia D, Nguyen Nhut Xuan D (2003) Food, feed or medicine: the multiple functions of edible wild plants in Vietnam. Econ Bot 57(1):103–117. https://doi.org/10.1663/0013-0001 Obata K, Kojima T, Masaki T, Okabayashi T, Yokota S, Hirakawa S, Nomura K, Takasawa A, Murata M, Tanaka S, Fuchimoto J, Fujii N, Tsutsumi H, Himi T, Sawada N (2013) Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS ONE 8(9):e70225. https://doi.org/10.1371/journal.pone.0070225 Prasad S, Aggarwal BB (2011) Turmeric, the golden spice: from traditional medicine to modern medicine. In: Benzie IFF, Wachtel-Galor S (eds) Herbal medicine: biomolecular and clinical aspects, 2nd edn. CRC Press/Taylor & Francis, Boca Raton Lattanzio V, Kroon PA, Linsalata V, Cardinali A (2009) Globe artichoke: a functional food and source of nutraceutical ingredients. J Funct Foods 1(2):131–144. https://doi.org/10.1016/j.jff.2009.01.002 Harish GU, Danapur V, Jain R, Patell VM (2012) Endangered Medicinal Plant Embelia ribes Burm. f.: a review. Pharmacogn J 4(27):6–19. https://doi.org/10.5530/pj.2012.27.2 Baliga MS, Bhat HP, Baliga BRV, Wilson R, Palatty PL (2011) Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam. (black plum): a review. Food Res Int 44(7):1776–1789. https://doi.org/10.1016/j.foodres.2011.02.007 Sood R, Swarup D, Bhatia S, Kulkarni D, Dey S, Saini M, Dubey S (2012) Antiviral activity of crude extracts of Eugenia jambolana Lam. against highlypathogenic avian influenza (H5N1) virus. Indian J Exp Biol 50(3):179–186 Belwal T, Giri L, Bahukhandi A, Tariq M, Kewlani P, Bhatt ID, Rawal RS, Nabavi SM, Silva AS (2019) Chapter 319- Ginkgo biloba. In: Nonvitamin and Nonmineral Nutritional Supplements. Academic Press, pp 241–250. Doi:https://doi.org/10.1016/B978-0-12-812491-8.00035-7 Thakur AK, Raj P (2017) Pharmacological perspective of Glycyrrhiza Glabra Linn.: a mini-review. J Anal Pharm Res 5(5):156. https://doi.org/10.15406/japlr.2017.05.00156 Hayashi H, Sudo H (2009) Economic importance of licorice. Plant Biotechnol 26:101–104. https://doi.org/10.5511/plantbiotechnology.26.101 Edell D, R.A H (2004) Herbal formulation of Gymnema sylvestre as a dietary aid. United States Patent Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L.: a phytochemical and pharmacological review. Food Chem 165:424–443. https://doi.org/10.1016/j.foodchem.2014.05.002 Baatartsogt T, Bui VN, Trinh DQ, Yamaguchi E, Gronsang D, Thampaisarn R, Ogawa H, Imai K (2016) High antiviral effects of hibiscus tea extract on the H5 subtypes of low and highly pathogenic avian influenza viruses. J Vet Med Sci 78(9):1405–1411. https://doi.org/10.1292/jvms.16-0124 Rajyalakshmi P, Venkatalaxmi K, Venkatalakshmamma K, Jyothsna Y, Balachandramani Devi K, Suneetha V (2001) Total carotenoid and beta-carotene contents of forest green leafy vegetables consumed by tribals of south India. Plant Foods Hum Nutr 56(3):225–238. https://doi.org/10.1023/a:1011125232097 Costa H, Ronchi S, Brasil G, Nascimento A, Lima E, Scherer R, Romão W, Boëchat G, Lenz D, Fronza M, Bissoli N, Endringer D, Andrade T (2015) Phytochemical and in vitro and in vivo biological investigation on the antihypertensive activity of mango leaves (Mangifera indica L.). Ther Adv Cardiovasc Dis 9(5):244–256. https://doi.org/10.1177/1753944715572958 MacLeod AJ, de Troconis NG (1982) Volatile flavour components of mango fruit. Phytochemistry 21(10):2523–2526. https://doi.org/10.1016/0031-9422(82)85249-7 Morton JF (1967) The balsam pear- an edible, medicinal and toxic plant. Econ Bot 21(1):57–68. https://doi.org/10.1007/bf02897176 Lee-Huang S, Huang PL, Chen HC, Huang PL, Bourinbaiar A, Huang HI, Kung HF (1995) Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene 161(2):151–156. https://doi.org/10.1016/0378-1119(95)00186-a Sánchez-Machado D, Núñez-Gastélum JA, Reyes Moreno C, Ramirez-Wong B, López-Cervantes J (2010) Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods 3(3):175–180. https://doi.org/10.1007/s12161-009-9106-z Nworu EL, Esimone CO, Ezeifeka CS, Okoye GO (2015) Extracts of Moringa oleifera Lam. showing inhibitory activity against early steps in the infectivity of HIV-1 lentiviral particles in a viral vector-based screening. Afr J Biotechnol 12:4866–4873. https://doi.org/10.5897/AJB2013.12343 Rawat S, Jugran A, Giri L, Bhatt I, Rawal R (2011) Assessment of antioxidant properties in fruits of Myrica esculenta: a popular wild edible species in indian Himalayan region. Evid Based Complement Alternat Med 2011:8. https://doi.org/10.1093/ecam/neq055 Makdoh K, Lynser MB, Pala KHM (2014) Marketing of indigenous fruits: a source of income among Khasi Women of Meghalaya, North East India. J Agric Sci 5:1–9. https://doi.org/10.1080/09766898.2014.11884707 Subratti A, Lalgee LJ, Jalsa NK (2019) Efficient extraction of black cumin (Nigella sativa L.) seed oil containing thymol, using liquefied dimethyl ether (DME). J Food Process Preserv 43(4):13913 Dubey PN, Singh B, Mishra B, Kant K, Solanki R (2016) Nigella (Nigella sativa): a high value seed spice with immense medicinal potential. Indian J Agric Sci 86:967–979 Cohen MM (2014) Tulsi-Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med 5(4):251–259. https://doi.org/10.4103/0975-9476.146554 Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N (2012) Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.): a review. Int J Mol Sci 13(3):3291–3340. https://doi.org/10.3390/ijms13033291 Broughton WJ, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Beans (Phaseolus spp.)-model food legumes. Plant Soil 252:55–128. https://doi.org/10.1023/A:1024146710611 Ihantola-Vormisto A, Summanen J, Kankaanranta H, Vuorela H, Asmawi M, Moilanen E (1998) Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med 63(6):518–524. https://doi.org/10.1055/s-2006-957754 Morton JF (1960) The emblic (Phyllunthus emblica L.). Econ Bot 14:119–128 Estari M, Venkanna L, Sripriya D, Lalitha R (2012) Human Immunodeficiency Virus (HIV-1) reverse transcriptase inhibitory activity of Phyllanthus emblica plant extract. Biol Med 4(4):178–182 Al-Maiman SA, Ahmad D (2002) Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem 76(4):437–441. https://doi.org/10.1016/S0308-8146(01)00301-6 Li Z, Wang K, Zheng J, Cheung FSG, Chan T, Zhu L, Zhou F (2014) Interactions of the active components of Punica granatum (pomegranate) with the essential renal and hepatic human Solute Carrier transporters. Pharm Biol 52(12):1510–1517 Moradi MT, Karimi A, Shahrani M, Hashemi L, Ghaffari-Goosheh MS (2019) Anti-Influenza virus activity and phenolic content of pomegranate (Punica granatum L.) peel extract and fractions. Avicenna J Med Biotechnol 11(4):285–291 Kuete V (2014) Chapter 22-physical, hematological, and histopathological signs of toxicity induced by African medicinal plants. In: Toxicological Survey of African Medicinal Plants. Elsevier, pp 635–657. https://doi.org/https://doi.org/10.1016/B978-0-12-800018-2.00022-4 Tavares IMdC, Lago-Vanzela ES, Rebello LPG, Ramos AM, Gómez-Alonso S, García-Romero E, Da-Silva R, Hermosín-Gutiérrez I (2016) Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels). Food Res Int 82:1–13. https://doi.org/10.1016/j.foodres.2016.01.014 Yang MH, Ali Z, Khan IA, Khan SI (2014) Anti-inflammatory activity of constituents isolated from Terminalia chebula. Nat Prod Commun 9(7):965–968 Barthakur NN, Arnold NP (1991) Nutritive value of the chebulic myrobalan (Terminalia chebula Retz) and its potential as a food source. Food Chem 40(2):213–219. https://doi.org/10.1016/0308-8146(91)90105-W Korani M, Jamshidi M (2020) The effect of aqueous extract of Trachyspermum ammi seeds and ibuprofen on inflammatory gene expression in the cartilage tissue of rats with collagen-induced arthritis. J Inflamm Res 13:133–139. https://doi.org/10.2147/jir.s236242 Srivastava KC (1988) Extract of a spice-Omum (Trachyspermum ammi)-shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins Leukot Essent Fatty Acids 33(1):1–6. https://doi.org/10.1016/0952-3278(88)90115-9 Cai Z, Zhang G, Tang B, Liu Y, Fu X, Zhang X (2015) Promising anti-influenza properties of active constituent of Withania somnifera ayurvedic herb in targeting neuraminidase of H1N1 influenza: computational study. Cell Biochem Biophys 72(3):727–739. https://doi.org/10.1007/s12013-015-0524-9 Palliyaguru DL, Singh SV, Kensler TW (2016) Withania somnifera: From prevention to treatment of cancer. Mol Nutr Food Res 60(6):1342–1353. https://doi.org/10.1002/mnfr.201500756 Chang JS, Wang KC, Yeh CF, Shieh DE, Chiang LC (2013) Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol 145(1):146–151. https://doi.org/10.1016/j.jep.2012.10.043 Semwal RB, Semwal DK, Combrinck S, Viljoen AM (2015) Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry 117:554–568. https://doi.org/10.1016/j.phytochem.2015.07.012 Nair KPP (2013) Chapter 26- Ginger as a Spice and Flavorant. In: The Agronomy and Economy of Turmeric and Ginger. Elsevier, Oxford, pp 497–510. doi:https://doi.org/10.1007/978-3-030-29189-1_26 Tripathi P, Chauhan NS, Patel JR (2012) Anti-inflammatory activity of Abutilon indicum extract. Nat Prod Res 26(17):1659–1661. https://doi.org/10.1080/14786419.2011.616508 Dashputre N, Naikwade N (2010) Immunomodulatory Activity of Abutilon Indicum linn on Albino Mice. Int J Pharm Sci Res 1(3):178–184 Bhuvaneswari K, Michael D (2000) Immunomodulation by leaf extract of Acalypha indica Linn in Oreochromis mossambicus (Peters). Hydrobiologia 430(1):113–120 Benni JM, Jayanthi MK, Suresha RN (2011) Evaluation of the anti-inflammatory activity of Aegle marmelos (Bilwa) root. Indian J Pharmacol 43(4):393–397. https://doi.org/10.4103/0253-7613.83108 Govinda HV, Asdaq SMB (2011) Immunomodulatory potential of methanol extract of Aegle marmelos in animals. Indian J Pharm Sci 73(2):235–240. https://doi.org/10.4103/0250-474x.91571 Kim JJ, Jiang J, Shim DW, Kwon SC, Kim TJ, Ye SK, Kim MK, Shin YK, Koppula S, Kang TB, Choi DK, Lee KH (2012) Anti-inflammatory and anti-allergic effects of Agrimonia pilosa Ledeb extract on murine cell lines and OVA-induced airway inflammation. J Ethnopharmacol 140(2):213–221. https://doi.org/10.1016/j.jep.2011.12.035 Lee DY, Li H, Lim HJ, Lee HJ, Jeon R, Ryu J-H (2012) Anti-inflammatory activity of sulfur-containing compounds from garlic. J Med Food 15(11):992–999. https://doi.org/10.1089/jmf.2012.2275 Fallah-Rostami F, Tabari MA, Esfandiari B, Aghajanzadeh H, Behzadi MY (2013) Immunomodulatory activity of aged garlic extract against implanted fibrosarcoma tumor in mice. N Am J Med Sci 5(3):207–212. https://doi.org/10.4103/1947-2714.109191 Ried K, Fakler P (2014) Potential of garlic (Allium sativum) in lowering high blood pressure: mechanisms of action and clinical relevance. Integr Blood Press Control 7:71–82. https://doi.org/10.2147/ibpc.s51434 Vázquez B, Avila G, Segura D, Escalante B (1996) Antiinflammatory activity of extracts from Aloe vera gel. J Ethnopharmacol 55(1):69–75. https://doi.org/10.1016/s0378-8741(96)01476-6 Farahnejad Z, Ghazanfari T, Yaraee R (2011) Immunomodulatory effects of Aloe vera and its fractions on response of macrophages against Candida albicans. Immunopharmacol Immunotoxicol 33(4):676–681. https://doi.org/10.3109/08923973.2011.560158 Yao W, Wang F, Wang H (2016) Immunomodulation of artemisinin and its derivatives. Sci Bull. https://doi.org/10.1007/s11434-016-1105-z Chattopadhyay RR (1998) Possible biochemical mode of anti-inflammatory action of Azadirachta indica A. Juss. in rats. Indian J Exp Biol 36(4):418–420 Upadhyay SN, Dhawan S, Garg S, Talwar GP (1992) Immunomodulatory effects of neem (Azadirachta indica) oil. Int J Immunopharmacol 14(7):1187–1193. https://doi.org/10.1016/0192-0561(92)90054-o Arise RO, Acho MA, Yekeen AA, Omokanye IA, Sunday-Nwaso EO, Akiode OS, Malomo SO (2019) Kinetics of angiotensin-1 converting enzyme inhibition and antioxidative properties of Azadirachta indica seed protein hydrolysates. Heliyon 5(5):e01747. https://doi.org/10.1016/j.heliyon.2019.e01747 Chen BT, Li WX, He RR, Li YF, Tsoi B, Zhai YJ, Kurihara H (2012) Anti-inflammatory effects of a polyphenols-rich extract from tea (Camellia sinensis) flowers in acute and chronic mice models. Oxid Med Cell Longev 2012:537923. https://doi.org/10.1155/2012/537923 Rahayu RP, Prasetyo RA, Purwanto DA, Kresnoadi U, Iskandar RPD, Rubianto M (2018) The immunomodulatory effect of green tea (Camellia sinensis) leaves extract on immunocompromised Wistar rats infected by Candida albicans. Vet World 11(6):765–770. https://doi.org/10.14202/vetworld.2018.765-770 Dong J, Xu X, Liang Y, Head R, Bennett L (2011) Inhibition of angiotensin converting enzyme (ACE) activity by polyphenols from tea (Camellia sinensis) and links to processing method. Food Funct 2(6):310–319. https://doi.org/10.1016/j.foodchem.2011.09.060 Gopakumar S, Latha P, Shine VJ, Anuja G, Suja S, ShyamaPeriyaRajasekharan SSPS (2010) Anti-allergic, anti-inflammatory and anti-lipidperoxidant effects of Cassia occidentalis Linn. Indian J Exp Biol 48(5):494–498 Doppalapudi SC, Sandya L, Reddy Y, Nagarjuna S, Shafeen S (2012) Anti-inflammatory activity of Cicer arietinum seed extracts. Asian J Pharm Clin Res 5:64–68 Sathyanarayana S, Kumar P, Prashanth H (2019) Pectic polysaccharides have relatively potent immunomodulatory activity compared to their hydrolysates from chickpea (Cicer arietinum L.) husk. Indian J Nutr Diet 56(2):94. https://doi.org/10.21048/ijnd.2019.56.2.22687 Bhagyawant SS, Narvekar DT, Gupta N, Bhadkaria A, Gautam AK, Srivastava N (2019) Chickpea (Cicer arietinum L.) lectin exhibit Inhibition of ACE-I, α-amylase and α-glucosidase activity. Protein Pept Lett 26(7):494–501. https://doi.org/10.2174/0929866526666190327130037 Mensah AY, Mireku EA, Damoah AO, Amponsah IK (2014) Anti-inflammatory and antioxidant activities of Commelina diffusa (Commelinaceae). World J Pharm Sci 2(10):1159–1165 Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14(2):141–153 Chandrasekaran CV, Sundarajan K, Edwin JR, Gururaja GM, Mundkinajeddu D, Agarwal A (2013) Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. J Pharm Pharmacogn Res 5(2):71–79. https://doi.org/10.4103/0974-8490.110527 Lekshmi PC, Arimboor R, Nisha VM, Menon AN, Raghu KG (2014) In vitro antidiabetic and inhibitory potential of turmeric (Curcuma longa L) rhizome against cellular and LDL oxidation and angiotensin converting enzyme. Int J Food Sci Technol 51(12):3910–3917. https://doi.org/10.1007/s13197-013-0953-7 Ben Salem M, Affes H, Athmouni K, Ksouda K, Dhouibi R, Sahnoun Z, Hammami S, Zeghal KM (2017) Chemicals compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid Based Complement Alternat Med 2017:4951937. https://doi.org/10.1155/2017/4951937 Chitra M, Sukumar E, Suja V, Devi CSS (1994) Antitumor, anti-inflammatory and analgesic property of Embelin, a plant product. Chemotherapy 40(2):109–113. https://doi.org/10.1159/000239181 Sethi J, Singh J (2015) Role of medicinal plants as immunostimulants in health and disease. Ann Med Chem Res 1(2):1009. https://doi.org/10.1016/j.fsi.2017.05.034 Kota P, Prasad PD, Rao AN, Reddy PD, Abhinay G (2010) Anti-inflammatory activity of Eugenia Jambolana in Albino rats. Int J Pharma Bio Sci 1(4):435–438 Mastan SK, Saraseeruha A, Gourishankar V, Chaitanya G, Raghunandan N, Reddy GA, Eswar Kumar KE (2008) Immunomodulatory activity of methanolic extract of Syzygium cumini seeds. Pharmacology online 3:895–903 Syama HP, Arya AD, Dhanya R, Nisha P, Sundaresan A, Jacob E, Jayamurthy P (2017) Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. J Food Sci Technol 54(7):2115–2125. https://doi.org/10.1007/s13197-017-2651-3 Kaur S, Sharma N, Nehru B (2018) Anti-inflammatory effects of Ginkgo biloba extract against trimethyltin-induced hippocampal neuronal injury. Inflammopharmacology 26(1):87–104. https://doi.org/10.1007/s10787-017-0396-2 Xu AH, Ren L, Zheng YY, Chen HS (2008) Immunomodulatory effect of Ginkgo biloba exocarp polysaccharides on immunosuppressive mice induced by cyclophosphamide. Chin J Pharmacol Toxicol 22(1):69–72 Ma FF, Wang H, Wei CK, Thakur K, Wei ZJ, Jiang L (2018) Three novel ACE inhibitory peptides isolated from Ginkgo biloba seeds: purification purification, inhibitory kinetic and mechanism. Front Pharmacol 9:1579. https://doi.org/10.3389/fphar.2018.01579 Frattaruolo L, Carullo G, Brindisi M, Mazzotta S, Bellissimo L, Rago V, Curcio R, Dolce V, Aiello F, Cappello AR (2019) Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants. PLoS ONE 8(6):186. https://doi.org/10.3390/antiox8060186 Mitra Mazumder P, Pattnayak S, Parvani H, Sasmal D, Rathinavelusamy P (2012) Evaluation of immunomodulatory activity of Glycyrhiza glabra L. roots in combination with zing. Asian Pac J Trop Biomed 2(1):S15–S20. https://doi.org/10.1016/S2221-1691(12)60122-1 Malik J, Manvi FV, Alagawadi KR, Noolvi M (2008) Evaluation of anti-inflammatory activity of Gymnema sylvestre leaves extract in rats. Int J Green Pharm. https://doi.org/10.4103/0973-8258.41184 Singh VK, Dwivedi P, Chaudhary BR, Singh R (2015) Immunomodulatory effect of gymnema sylvestre (RBr). leaf extract: an in vitro study in rat model. PLoS ONE 10(10):0139631. https://doi.org/10.1371/journal.pone.0139631 Bayani GFE, Marpaung NLE, Simorangkir DAS, Sianipar IR, Ibrahim N, Kartinah NT, Mansur IG, Purba JS, Ilyas EII (2018) Anti-inflammatory effects of Hibiscus Sabdariffa Linn. on the IL-1β/IL-1ra ratio in plasma and hippocampus of overtrained rats and correlation with spatial memory. Kobe J Med Sci 64(2):E73–E83 Fakeye TO, Pal A, Bawankule DU, Khanuja SP (2008) Immunomodulatory effect of extracts of Hibiscus sabdariffa L. (Family Malvaceae) in a mouse model. Phytother Res 22(5):664–648. https://doi.org/10.1002/ptr.2370 Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L (2010) Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol 127(1):7–10. https://doi.org/10.1016/j.jep.2009.09.059 Patil N, Kotian R, Reddy S, Nayak V, Bairy L, Parida A, Malalur C (2014) Evaluation of anti-inflammatory activity of alcoholic extract of leaves of Leucas Aspera in albino rats. Int J Pharm Pharm Sci 6(2) Augustine B, Dash S, Lahkar M, Amara V, Samudrala P, Thomas J (2014) Evaluation of immunomodulatory activity of ethyl acetate extract of Leucas aspera in Swiss albino mice. Int J Green Pharm 8(2):84. https://doi.org/10.4103/0973-8258.129574 Knödler M, Jr C, Wenzig EM, Bauer R, Lacorn M, Beifuss U, Carle R, Schieber A (2008) Anti-inflammatory 5-(11’Z-heptadecenyl)- and 5-(8’Z,11’Z-heptadecadienyl)-resorcinols from mango (Mangifera indica L.) peels. Phytochemistry 69(4):988–993. https://doi.org/10.1016/j.phytochem.2007.10.013 Makare N, Bodhankar S, Rangari V (2001) Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol 78(2):133–137. https://doi.org/10.1016/S0378-8741(01)00326-9 Juvekar A, Hule A, Sakat S, Chaughule V (2009) In vitro and in vivo evaluation of immunomodulatory activity of methanol extract of Momordica charantia fruits. Drug Invent Today 1(2):89–94 Priyanto AD, Doerksen RJ, Chang CI, Sung W-C, Widjanarko SB, Kusnadi J, Lin YC, Wang TC, Hsu JL (2015) Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. J Proteom 128:424–435. https://doi.org/10.1016/j.jprot.2015.08.018 Anudeep S, Prasanna VK, Adya SM, Radha C (2016) Characterization of soluble dietary fiber from Moringa oleifera seeds and its immunomodulatory effects. Int J Biol Macromol 91:656–662. https://doi.org/10.1016/j.ijbiomac.2016.06.013 Abdulazeez A, Ajiboye O, Wudil A, Abubakar H (2016) Partial purification and characterization of angiotensin converting enzyme inhibitory alkaloids and flavonoids from the leaves and seeds of Moringa oleifera. J adv biol biotechnol 5:1–11. https://doi.org/10.9734/jabb/2016/21974 Patel KG, Rao NJ, Gajera VG, Bhatt PA, Patel KV, Gandhi TR (2010) Anti-allergic activity of stem bark of Myrica esculenta Buch-Ham (Myricaceae). J Young Pharm 2(1):74–78. https://doi.org/10.4103/0975-1483.62219 Nguyen XN, Phan VK, Chau VM, Bui HT, Nguyen XC, Vu KT, le Hoang TA, Jo SH, Jang HD, Kwon YI, Kim YH (2010) A new monoterpenoid glycoside from Myrica esculenta and the inhibition of Angiotensin I-converting enzyme. Chem Pharm Bull 58:1408–1410. https://doi.org/10.1248/cpb.58.1408 Ikhsan M, Hiedayati N, Maeyama K, Nurwidya F (2018) Nigella sativa as an anti-inflammatory agent in asthma. BMC Res Notes 11(1):744–744. https://doi.org/10.1186/s13104-018-3858-8 Boskabady MH, Keyhanmanesh R, Khameneh S, Doostdar Y, Khakzad MR (2011) Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Univ Sci B 12(3):201–209. https://doi.org/10.1631/jzus.B1000163 Sutopo CCY, Sutrisno A, Wang LF, Hsu JL (2020) Identification of a potent Angiotensin-I converting enzyme inhibitory peptide from Black cumin seed hydrolysate using orthogonal bioassay-guided fractionations coupled with in silico screening. Process Biochem. https://doi.org/10.1016/j.procbio.2020.02.010 Kalabharathi HL, Suresha RN, Pragathi B, Pushpa VH, Satish AM (2011) Anti inflammatory activity of fresh tulsi leaves (Ocimum Sanctum) in albino rats. Int J Pharma Bio Sci 2(4):45–50 Mediratta PK, Sharma KK, Singh S (2002) Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol 80(1):15–20. https://doi.org/10.1016/s0378-8741(01)00373-7 Chaudhary S, Mukherjee P, Maity N, Nema N, Bhadra S, Saha BP (2014) Ocimum sanctum L. a potential angiotensin converting enzyme (ACE) inhibitor useful in hypertension. Indian J Nat Prod Resour 5(1):83–87 Song C, Hong YH, Park JG, Kim HG, Jeong D, Oh J, Sung G-H, Hossain MA, Taamalli A, Kim JH, Kim J-H, Cho JY (2019) Suppression of Src and Syk in the NF-ÎoB signaling pathway by Olea europaea methanol extract is leading to its anti-inflammatory effects. J Ethnopharmacol 235:38–46. https://doi.org/10.1016/j.jep.2019.01.024 Vezza T, Algieri F, Rodríguez-Nogales A, Garrido-Mesa J, Utrilla MP, Talhaoui N, Gómez-Caravaca AM, Segura-Carretero A, Rodríguez-Cabezas ME, Monteleone G, Gálvez J (2017) Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol Nutr Food Res 61(10) Hansen K, Adsersen A, Christensen SB, Jensen SR, Nyman U, Smitt UW (1996) Isolation of an angiotensin converting enzyme (ACE) inhibitor from Olea europaea and Olea lancea. Phytomedicine 2(4):319–325. https://doi.org/10.1016/S0944-7113(96)80076-6 Oomah BD, Corbé A, Balasubramanian P (2010) Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J Agric Food Chem 58(14):8225–8230. https://doi.org/10.1021/jf1011193 Tagliazucchi D, Martini S, Bellesia A, Conte A (2015) Identification of ACE-inhibitory peptides from Phaseolus vulgaris after in vitro gastrointestinal digestion. Int J Food Sci Nutr 66(7):774–782. https://doi.org/10.3109/09637486.2015.1088940 Suresh K, Vasudevan DM (1994) Augmentation of murine natural killer cell and antibody dependent cellular cytotoxicity activities by Phyllanthus emblica, a new immunomodulator. J Ethnopharmacol 44:55–60. https://doi.org/10.1016/0378-8741(94)90099-x Lee CJ, Chen LG, Liang WL, Wang CC (2010) Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem 118(2):315–322. https://doi.org/10.1016/j.foodchem.2009.04.123 Gracious Ross R, Selvasubramanian S, Jayasundar S (2001) Immunomodulatory activity of Punica granatum in rabbits: a preliminary study. J Ethnopharmacol 78(1):85–87. https://doi.org/10.1016/s0378-8741(01)00287-2 Wang Y, **ang L, Yi X, He X (2017) Potential anti-inflammatory steroidal saponins from the berries of Solanum nigrum L. (European Black Nightshade). J Agric Food Chem 65(21):4262–4272. https://doi.org/10.1021/acs.jafc.7b00985 Li J, Li QW, Gao DW, Han ZS, Lu WZ (2009) Antitumor and immunomodulating effects of polysaccharides isolated from Solanum nigrum Linne. Phytother Res 23(11):1524–1530. https://doi.org/10.1002/ptr.2769 Chaudhuri AKN, Pal S, Gomes A, Bhattacharya S (1990) Anti-inflammatory and related actions of Syzygium cuminii seed extract. Phytother Res 4(1):5–10. https://doi.org/10.1002/ptr.2650040103 Shivaprasad HN, Kharya MD, Rana AC, Mohan S (2006) Preliminary immunomodulatory activities of the aqueous extract of Terminalia chebula. Pharm Biol 44(1):32–34. https://doi.org/10.1080/13880200500530542 Sornwatana T, Bangphoomi K, Roytrakul S, Wetprasit N, Choowongkomon K, Ratanapo S (2015) Chebulin: Terminalia chebula Retz. fruit-derived peptide with angiotensin-I-converting enzyme inhibitory activity. Biotechnol Appl Biochem 62(6):746–753. https://doi.org/10.1002/bab.1321 Shruthi RR, Venkatesh Y, Gudipati M (2017) In vitro immunomodulatory potential of macromolecular components derived from the aqueous extract of ajowan [Trachyspermum ammi (L.) Sprague]. Indian J Tradit Know 16(3):506–513 Gupta A, Singh S (2014) Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharm Biol 52(3):308–320. https://doi.org/10.1186/s12906-016-1466-5 Davis L, Kuttan G (2000) Immunomodulatory activity of Withania somnifera. J Ethnopharmacol 71(1–2):193–200. https://doi.org/10.1016/s0378-8741(99)00206-8 Ravindran R, Sharma N, Roy S, Thakur A, Ganesh S, Kumar S, Devi J, Rajkumar J (2015) Interaction studies of Withania somnifera’s key metabolite withaferin a with different receptors associated with cardiovascular disease. Curr Comput Aided Drug Des 11(3):212–221. https://doi.org/10.2174/1573409912666151106115848 Funk JL, Frye JB, Oyarzo JN, Chen J, Zhang H, Timmermann BN (2016) Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. Pharmanutrition 4(3):123–131. https://doi.org/10.1016/j.phanu.2016.02.004 Amri M, Touil-Boukoffa C (2016) In vitro anti-hydatic and immunomodulatory effects of ginger and [6]-gingerol. Asian Pac J Trop Med 9(8):749–756. https://doi.org/10.1016/j.apjtm.2016.06.013 Akinyemi A, Ademiluyi A, Oboh G (2014) Inhibition of angiotensin-1-converting enzyme activity by two varieties of ginger ( Zingiber officinale ) in rats fed a high cholesterol diet. J Med Food. https://doi.org/10.1089/jmf.2012.0264References
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, B., Sharma, S., Nair, N. et al. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol Cell Biochem 476, 2345–2364 (2021). https://doi.org/10.1007/s11010-021-04084-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04084-7