Abstract
Several acridine derivatives have been screened for their therapeutic potential and some are already established as antiprotozoan, antibacterial or anticancer agents. However, phenyl derivative at C-9 position of acridine had remained unexplored for their biological activity so far. In this report, we present our findings with 9-phenyl acridine (ACPH) as an anticancer agent. Both A375 and HeLa, two human cancer cell lines, were more sensitive to ACPH than normal cells namely human lymphocytes and also Chinese hamster V79 cells. ACPH also led to regression of tumour volume in mice. In A375 cells, we have shown that it caused DNA damage and blocked cell cycle progression at G2-M phase. Treatment with ACPH led to lowering of mitochondrial potential, upregulation of bax, release of cytochrome C and activation of caspase 3. As a new agent showing lower toxicity to normal cells and greater sensitivity towards cancerous cell line, ACPH shows promise as an effective cancer chemotherapeutic agent. ACPH treatment resulted in apoptotic death of cells through mitochondria-mediated caspase-dependent pathway.






Similar content being viewed by others
Abbreviations
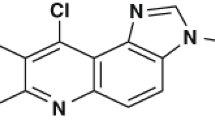
- ACPH:
-
9-Phenyl acridine
- Topo I:
-
Topoisomerase I
- ΔΨ mt :
-
Mitochondrial membrane potential
- PS:
-
Phophatidylserine
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labelling
- Rh123:
-
Rhodamine 123
References
Belmont P, Bosson J, Godet T, Tiano M (2007) Acridine and acridone derivatives, anticancer properties and synthetic methods: where are we now. Anticancer Agents Med Chem 7:139–169
Hurwitz J, Furth JJ, Malamy M, Alexander M (1962) The role of deoxyribonucleic acid in ribonucleic acid synthesis, III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci USA 48:1222–1230
Cate JH, Doudna JA (1996) Metal-binding sites in the major groove of a large ribozyme domain. Structure 4:1221–1229
Rueda M, Luque FJ, Orozco M (2005) Nature of minor-groove binders—DNA complexes in the gas phase. J Am Chem Soc 127:11690–11698
Turner PR, Denny WA (1996) The mutagenic properties of DNA minor-groove binding ligands. Mutat Res 355:141–169
Kohn KW (1996) Beyond DNA cross-linking: history and prospects of DNA targeted cancer treatment-fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res 56:5533–5546
Harris CC (1996) Structure and function of p53 tumor suppressor gene: clues for rational cancer therapeutic strategies. J Natl Cancer Inst 88:1442–1455
Wesierska-Gadek J, Schloffer D, Gueorguieva M, Uhl M, Skladanowski A (2004) Increased susceptibility of poly(ADP-ribose) polymerase-1 knockout cells to antitumour triazoloacridone C-1305 is associated with permanent G2 cell cycle arrest. Cancer Res 64:4487–4497
Ghosh R, Bhowmik S, Bagchi A, Das D, Ghosh S (2010) Chemotherapeutic potential of 9-phenyl acridine: biophysical studies on its binding to DNA. Eur Biophys J 39:1243–1249
Yamori T, Matsunaga A, Sato S, Yamazaki K, Komi A, Ishizu K, Mita I et al (1999) Potent Antitumour activity of MS-247, a novel DNA minor groove binder evaluated by an in vitro and in vivo human cancer cell line panel1. Cancer Res 59:4042–4049
Bhuyan BK, Smith KS, Adams EG, Wallace TL, Von Hoff DD, Li LH (1992) Adozelesin, a potent new alkylating agent: cell-killing kinetics and cell-cycle effects. Cancer Chemother Pharmacol 30:348–354
Hisiang YH, Hertzberg R, Hecht S, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260:14873–14878
Sausville EA, Burger AM (2006) Contributions of human tumor xenografts to anticancer drug development. Cancer Res 66:3351–3354
Sordet O, Liao Z, Liu H, Antony S, Stevens EV, Kohlhagen G, Fu H, Pommier Y (2004) Topoisomerase I-DNA Complexes Contribute to Arsenic Trioxide-induced apoptosis. J Biol Chem 279:33968–33975
Bernardi P, Petronilli V (1996) The permeability transition pore as a mitochondrial calcium release channel: a critical appraisal. J Bioenerg Biomembr 28:129–136
Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG (2003) Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett 190:157–163
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programed cell death. Cell 74:609–619
Aggarwal S, Taneja N, Lin L, Orringer MB, Rehemtulla A, Beer DG (2000) Indomethacin-induced apoptosis esophageal adenocarcinoma cells involve upregulation of bax and translocation of mitochondrial cytochrome C independent of COX-2 expression. Neoplasia 2:346–356
Wolter KG, Hsu YT, Smith CL, Nechushtan A, ** XG, Youle RJ (1997) Movement of bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292
Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochrome C. Nature 391:496–499
Jurgensmeier JM, **e Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC (1998) Bax directly induces release of cytochrome C from isolated mitochondria. Proc Natl Acad Sci USA 95:4997–5002
Zamzami N, Marchetti P, Castedo M, Zamin C, Vayssiere JL, Petit PX, Kroemer G (1995) Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 181:1661–1672
Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A et al (1996) Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med 184:1155–1160
Chiu CC, Chang HW, Chuang DW, Chang FR, Chang YC, Cheng YS, Tsai MT et al (2009) Fern plant-derived protoapigenone leads to DNA damage, apoptosis, and G(2)/m arrest in lung cancer cell line H1299. DNA Cell Biol 28:501–506
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ et al (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48:589–601
Bajpayee M, Dhawan A, Parmar D, Pandey AK, Mathur N, Seth PK (2002) Gender related differences in basal DNA damage in lymphocytes of healthy Indian population as revealed by the alkaline Comet assay. Mutat Res 520:83–91
Wiklund SJ, Agruell E (2003) Aspects of design and statistical analysis in the Comet assay. Mutagenesis 18:167–175
Darzynkiewicz Z, Galkowski D, Zhao H (2008) Analysis of apoptosis by cytometry using TUNEL assay. Methods 44:250–254
Budzowska M, Jaspers I, Essers J, de Waard H, van Drunen E, Hanada K, Beverloo B, W. Hendriks R, de Klein A, Kanaar R, Hoeijmakers JH, Maas A (2004) Mutation of the mouse Rad17 gene leads to embryonic lethality and reveals a role in DNA damage-dependent recombination. EMBO J 23:3548-3558
Ito K, Nakazato T, Miyakawa Y, Yamato K, Ikeda Y, Kizaki M (2003) Caffeine induces G2/M arrest and apoptosis via a novel p53- dependent pathway in NB4 promyelocytic leukemia cells. J Cell Physiol 196:276–283
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Pre′vost MC et al (1999) Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med 189:381–393
van-Engeland M, Nieland LJW, Ramaekers FCS, Schutte B, Reutelingsperger PM (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1–9
Ghosh R, Girigoswami K (2008) NADH dehydrogenase subunits are overexpressed in cells exposed repeatedly to H2O2. Mutat Res 638:210–215
Huang J, Wu L, Tashiro S, Onodera S, Ikejima T (2008) Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci 107:370–379
Motiwale L, Ingle AD, Rao KV (2005) Mouse skin tumor promotion by sodium arsenate is associated with enhanced PCNA expression. Cancer Lett 223:27–35
Manoharan S, Muneeswaran M, Baskaran N (2010) Chemopreventive efficacy of berberine in 7, 12-dimethylbenz[a]anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. Int J Res Pharm Sci 1:521–529
Bhowmik S, Bagchi A, Ghosh R (2008) Molecular modelling studies of some 9-arylacridines to elucidate their possible roles in topoisomerase I inhibition. Int J Integrative Biol 2:8–14
Kluza J, Lansiaux A, Wattez N, Mahiew C, Osheroff N, Bailly C (2000) Apoptotic Response of HL-60 Human Leukemia Cells to the Antitumour Drud TAS-1031. Cancer Res 60:4077–4084
Kuo PL, Hsu YL, Cho CY (2006) Plumbagin induces G2-M arrest and Autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther 5:3209–3221
Ahmed N, Adhami VM, Afaq F, Feyes DK, Mukhtar H (2001) Resveratrol causes WAF-1/p21-mediated G1-phase arrest of cell cycle and induction of apoptosis in Human Epidermoid Carcinoma A431 Cells1. Clin Cancer Res 7:1466–1473
Chiu SM, Xue LY, Usuda J, Azizuddin K, Oleinick NL (2003) Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Br J Cancer 89:1590–1597
Naito M, Nagashima K, Mashima T, Tsuruo T (1997) Phosphatidylserine externalization is a downstream event of interleukin-1b-converting enzyme family protease activation during apoptosis. Blood 89:2060–2066
Stepczynska A, Lauber K, Engels IH, Janssen O, Kabelitz D, Wesselborg S, Schulze-Osthoff K (2001) Staurosporine and conventional anticancer drugs induce overlap**, yet distinct pathways of apoptosis and caspase activation. Oncogene 20:1193–1202
Acknowledgments
Sudipta Bhowmik is supported with the University Research Scholarship from University of Kalyani (KU) and Dipanjan Guha is a recipient of UGC-RFMS scholarship, Govt. of India. The authors acknowledge instrumental facilities at the Dept. Biochemistry & Biophysics, KU funded by DST-FIST, Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, R., Bhowmik, S. & Guha, D. 9-Phenyl acridine exhibits antitumour activity by inducing apoptosis in A375 cells. Mol Cell Biochem 361, 55–66 (2012). https://doi.org/10.1007/s11010-011-1088-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1088-7