Abstract
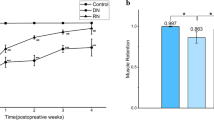
Myostatin, a member of the transforming growth factor-β (TGF-β) superfamily, has been identified as an inhibitor of skeletal muscle mass. To have an insight into the expression pattern of myostatin and its potential role in skeletal muscle atrophy induced by denervation, we used an animal model of peripheral nerve resection to examine the time-dependent changes in myostatin mRNA and protein levels in the denervated gastrocnemius muscle of rats after sciatic neurectomy by the aid of quantitative real-time RT-PCR and Western blotting, respectively. We also conducted morphometric analyses to measure the wet weight ratio of the denervated muscle (the operated side/contralateral non-operated side) and the cross sectional area of muscle fibers and to observe muscle morphology. The experimental results showed that myostatin mRNA and protein levels in rat gastrocnemius muscle persistently elevated after denervation, despite a fluctuation of myostatin mRNA level at day 3 after denervation, reached their respective peaks at day 28 after denervation, and then depressed slightly until day 56 after denervation. Furthermore, a significant negative linear correlation was found between myostatin abundance and muscle atrophy degree, suggesting that myostatin might probably play an important role in denervation-induced muscle atrophy. Our present study perhaps provides a new window into myostatin regulation in association with a specific type of muscle atrophy.
Similar content being viewed by others
References
Armand A-S, Gaspera BD, Launay T, Charbonnier F, Gallien CL, Chanoine C, (2003) Expression and neural control of follistatin versus myostatin genes during regeneration of mouse soleus Dev Dyn 227:256–265
Baumann AP, Ibebunjo C, Grasser WA, Paralkar VM, (2003) Myostatin expression in age and denervation-induced skeletal muscle atrophy J Musculoskelet Neuronal Interact 3:8–16
Carlson CJ, Booth FW, Gordon SE, (1999) Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading Am J Physiol Regul Integr Comp Physiol 277:R601–R606
Evans GRD, Brandt K, Niederbichler AD, Chauvin P, Hermann S, Bogle M, (2000) Clinical long-term in vivo evaluation of poly (L-lactic acid) porous conduits for peripheral nerve regeneration J Biomater Sci-Polym Ed 11:869–878
Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S, (1998) Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting Proc Natl Acad Sci USA 95:14938–14943
Grobet L, Martin LJR, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset Rn &Georges M, (1997) A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle Nat Gene 17:71–74
Heid CA, Stevens J, Livak KJ, Williams PM, (1996) Real-time quantitative PCR Genome Res 6:986–994
Holland PM, Abramson RD, Watson R, Gelfand DH, (1991) Detection of specific polymerase chain reaction product by utilizing the 5′-3′exonoclease to thermos aquaticus DNA polymerase Proc Natl Acad Sci USA 88:7276–7296
Jackman RW, Kandarian SC, (2004) The molecular basis of skeletal muscle atrophy Am J Physiol Cell Physiol 287: C834–C843
Johnson DR, Lee PKH, Holmes VF, Alvarez-Cohen L, (2005) An internal reference technique for accurately quantifying specific mRNAs by real-time PCR with application to the tceA reductive dehalogenase gene Appl Environ Microb 71:3866–3871
Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N and Greenhaff P (2004) Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass FASEB J. express article aoi: 10.1096/ fj.03-1228 fje, published online
Kambadur R, Sharma M, Smith TPL, Bass JJ, (1997) Mutations of myostatin (GDF-8) in double-muscled Belgian Blue and Piedmontese cattle Genome Res 7:910–916
Lalani R, Bhasin S, Byhower F, Tarnuzzer R, Grant M, Shen R, Asa S, Ezzat S, Gonzalez-Cadavid NF, (2000) Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight J Endocrinol 167:417–428
Lupberger J, Kreuzer K-A, Baskaynak G, Peters UR, Coutre PL, Schmidt CA, (2002) Quantitative analysis of beta-actin, beta-2-microglobulin and porphobilinogen deaminase mRNA and their comparison as control transcripts for RT-PCR Mol Cell Probe 16:25–30
Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid NF, Arias J, Salehian B, (2003) Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression Am J Physiol Endocrinol Metab 285:E363–E371
McMahon CD, Popovic L, Oldham JM, Jeanplong F, Smith HK, Kambadur R, Shrma M, Maxwell L, Bass JJ, (2003) Myostatin-deficient mice lose more skeletal muscle mass than wild-type controls during hindlimb suspension Am J Physiol Endocrinol Metab 285:E82–E87
McPherron AC, Lawler AM, Lee S-J, (1997) Regulation of skeletal muscle mass in mice by a new TGF- ß superfamily member Nature 387:83–90
McPherron AC, Lee SJ, (1997) Double muscling in cattle due to mutations in the myostatin gene Proc Natl Acad Sci USA 94:12457–12461
Reardon K, Davis J, Kapsa R, Choong P, Byrne E, (2001) Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy Muscle Nerve 24:893–899
Roberts SB, Goetz FW, (2003) Myostatin protein and mRNA transcript levels in adult and develo** brook trout Mol Cell Endocrinol 210:9–20
Roberts SB, McCauley LAR, Devlin RH, Goetz FW, (2004) Transgenic salmon over-expressing growth hormone exhibit decreased myostatin transcript and protein expression J Exp Biol 207:3741–3748
Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T, (2000) Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles Biochim Biophys Acta 1497:77–88
Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R, (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation J Biol Chem 275:40235–40243
Wehling M, Cai B, Tidball JG, (2000) Modulation of myostatin expression during modified muscle use FASEB J 14:103–110
Zachwieja JJ, Smith SR, Sinha-Hikim I, Gonzalez-Cadavid NF, Bhasin S, (1999) Plasma myostatin-immunoreactive protein is increased after prolonged bed rest with low-dose T3 administration J Gravit Physiol 6:11–15
Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee S-J, (2002) Induction of cachexia in mice by systemically administered myostatin Science 296:1486–1488
Acknowledgements
We wish to thank professor Jie Liu for assistance in the preparation of this manuscript. This work was supported by Hi-Tech Research and Development Program of China (863 Program, Grant No. 2003AA205030) and National Natural Science Foundation of China (Grant No. 30270427).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Liu, M., Ding, F. et al. Expression of myostatin RNA transcript and protein in gastrocnemius muscle of rats after sciatic nerve resection. J Muscle Res Cell Motil 27, 37–44 (2006). https://doi.org/10.1007/s10974-005-9050-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-005-9050-5