Abstract
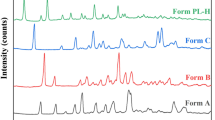
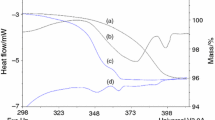
Levofloxacin (LF), a pure levo-isomer of ofloxacin, is a quinolone-class antibiotic marketed in its hemihydrate (LF-½H) crystal form. Another LF hydrate is known as monohydrate (LF-1H), and LF-½H and LF-1H dehydration results in a pair of anhydrous polymorphs: LF-γ (from LF-½H) and LF-α (from LF-1H). Herein, a pure crystalline material of each of these four LF crystal forms has been successfully produced and systematically characterized by X-ray powder diffraction (PXRD), infrared attenuated total reflectance spectroscopy, differential scanning calorimetry (DSC) and thermogravimetric analyses. Coupling cyclic DSC and ex-situ PXRD analyses allowed probing dehydration, melting, crystallization and polymorphic phase transitions involving LF-½H, LF-1H, LF-α, LF-γ, and LF-δ (an enantiotropic polymorph of LF-γ). Furthermore, for the first time, the LF-½H, LF-1H, LF-γ and LF-α equilibrium solubilities were individually measured in five different aqueous media (pH from 1.0 to 7.2). The general solubility order is: LF-γ > LF-½H = LF-α > LF-1H. The crystal phases identified in the residual solid materials separated from equilibrium solutions show that LF-½H and LF-α forms in converted to LF-1H. The information provided herein about stability and solubility of known LF crystal forms proved to be essential to control the hydration/dehydration/rehydration process between the LF crystalline phases and ensure safe drugs with low toxicity or design LF solids with properties improved physical chemistry.
Graphical Abstract










Similar content being viewed by others
References
Surov AO, Vasilev NA, Churakov AV, Parashchuk OD, Artobolevskii SV, Alatortsev OA, et al. Two faces of water in the formation and stabilization of multicomponent crystals of zwitterionic drug-like compounds. Symmetry (Basel). 2021;425:14.
Basford PA, Back KR, Cram M, Docherty R, Davey RJ, Cruz-Cabeza AJ. Impact of crystal structure and molecular conformation on the hydration kinetics of fluconazole. Cryst Growth Des. 2019;19:7193–205.
Chow K, Tong HHY, Lum S, Chow AHL. Engineering of pharmaceutical materials: an industrial perspective. J Pharm Sci. 2008;97:2855–77.
Stahly GP. Diversity in single and multiple-component crystals the search for and prevalence of polymorphs and cocrystals. Cryst Growth Des. 2007;7:1007–26.
Jurczak E, Mazurek AH, Szeleszczuk Ł, Pisklak DM, Zielińska-Pisklak M. Pharmaceutical hydrates analysis—overview of methods and recent advances. Pharmaceutics. 2020;12(10):959.
Ma X, Müller F, Huang S, Michael Lowinger X, Liu RS, Williams RO. Influence of carbamazepine dihydrate on the preparation of amorphous solid dispersions by hot melt extrusion. Pharmaceutics. 2020;12(4):379. https://doi.org/10.3390/pharmaceutics12040379.
Griesser UJ. In polymorphism. KGaA: Wiley-VCH Verlag GmbH & Co; 2006.
Braun DE, Griesser UJ. Stoichiometric and nonstoichiometric hydrates of brucine. Cryst Growth Des. 2016;16:6111–21.
Cavallari C, Fini A, Santos B. Thermal study of anhydrous and hydrated forms of olanzapine. Pharm Anal Acta. 2013;4(5):237–44.
Khankari RK, Grant DJW. Pharmaceutical hydrates. Thermochim Acta. 1995;248:61–79.
Roy S, Quiñones R, Matzger AJ. Structural and physicochemical aspects of dasatinib hydrate and anhydrate phases. Cryst Growth Des. 2012;12:2122–6.
Gift AD, Luner PE, Luedeman L, Taylor LS. Influence of polymeric excipients on crystal hydrate formation kinetics in aqueous slurries. J Pharm Sci. 2008;97:5198–211.
Vippagunta SR, Brittain HG, Grant DJW. Crystalline solids. Adv Drug Deliv Rev. 2001. https://doi.org/10.1016/S0169-409X(01)00097-7.
Grothe E, Meekes H, Vlieg E, Ter Horst JH, De Gelder R. Solvates, salts, and cocrystals: a proposal for a feasible classification system. Cryst Growth Des. 2016;16:3237–43.
Cruz-Cabeza AJ, Bernstein J. Conformational polymorphism. Chem Rev. 2014;114:2170–91.
Cruz-Cabeza AJ, Reutzel-Edens SM, Bernstein J. Facts and fictions about polymorphism. Chem Soc Rev. 2015;44(23):8619–35. https://doi.org/10.1039/C5CS00227C.
Borghetti GS, Carini JP, Honorato SB, Ayala AP, Moreira JCF, Bassani VL. Physicochemical properties and thermal stability of quercetin hydrates in the solid state. Thermochim Acta. 2012;539:109–14.
Giron D, Goldbronn C, Mutz M, Pfeffer S, Schwab P. Solid state characterizations of pharmaceutical hydrates. J Therm Anal Calorim. 2002;68:453–65.
Vardanyan R, Hruby V. Antibacterial drugs. In: Synthesis of best-seller drugs. Elsevier; 2016. p. 645–67. https://doi.org/10.1016/B978-0-12-411492-0.00031-6.
Kitaoka H, Wada C, Moroi R, Hakusui H. Effect of dehydration om the formation of levofloxacin pseudopolymorphs. Chem Pharm Bull. 1995;43:649–53.
Gorman EM, Samas B, Munson EJ. Understanding the dehydration of levofloxacin hemihydrate. J Pharm Sci. 2012;101:3319–30.
Singh SS, Thakur TS. New crystalline salt forms of levofloxacin: Conformational analysis and attempts towards the crystal structure prediction of the anhydrous form. CrystEngComm. 2014;16:4215–30.
Pereira RN, Fandaruff C, Riekes MK, Monti GA, Maduro CE, de Campos S, Cuffini L, Silva MAS. Grinding effect on levofloxacin hemihydrate. J Therm Anal Calorim. 2015;119(2):989–94. https://doi.org/10.1007/s10973-014-4233-1.
Freitas JTJ, De Melo CC, Viana OMMS, Ferreira FF, Doriguetto AC. Crystal structure of levofloxacin anhydrates: a high-temperature powder X-ray diffraction study versus crystal structure prediction. Cryst Growth Des. 2018;18:3558–68.
Thakur TS. Comment on “Polymorphism of levofloxacin: structure, properties and phase transformation”, CrystEngComm, by N. Wei, L. Jia, Z. Shang, J. Gong, S. Wu, J. Wang and W. Tang, 2019, 21, 6196–6207. CrystEngComm. 2020;22(10):1885–8.
Thakur TS. Reply to the “Comment on ‘polymorphism of levofloxacin: Structure, properties and phase transformation’” by Tejender S. Thakur. CrystEngComm. 2020;22(10):1885–8. https://doi.org/10.1039/C9CE01400D.
Wei N, Jia L, Shang Z, Gong J, Wu S, Wang J, et al. Polymorphism of levofloxacin: structure, properties and phase transformation. CrystEngComm. 2019;21:6196–207.
FDA. U.S. Food and Drug Administration. Levaquin® (levofloxacin). Highlights of prescribing information. 2008 [cited 2023 Jun 21]. p. 1–65. Available from: https://www.fda.gov/downloads/drugs/emergencypreparedness/bioterrorismanddrugpreparedness/ucm133684.pdf.
WHO - World Health Organization. Notes on the Design of Bioequivalence Study: Levofloxacin. https://extranet.who.int/pqweb/sites/default/files/documents/BE_levofloxacin_July2021.pdf. 2021.
WHO Prequalification of Medicines Programme - Guidance Document. Biopharmaceutics Classification System (BCS)-based biowaiver applications: anti-tuberculosis medicines. 2009;21.
Koeppe MO, Cristofoletti R, Fernandes EF, Storpirtis S, Junginger HE, Kopp S, Midha KK, Shah VP, Stavchansky S, Dressman JB, Barends DM. Biowaiver monographs for immediate release solid oral dosage forms: Levofloxacin. J Pharm Sci. 2011;100(5):1628–36.
Kłosińska-Szmurło E, Grudzień M, Betlejewska-Kielak K, Pluciński F, Biernacka J, Mazurek AP. Physicochemical properties of lomefloxacin, levofloxacin, and moxifloxacin relevant to the biopharmaceutics classification system. Acta Chim Slov. 2014;61:827–34.
O’Neil MJ; The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, editor. Whitehouse Station, NJ: NJ: Merck and Co., Inc.; 2006.
Frick A, Möller H, Wirbitzki E. Biopharmaceutical characterization of oral immediate release drug products. In vitro/in vivo comparison of phenoxymethylpenicillin potassium, glimepiride and levofloxacin. Eur J Pharm Biopharm. 1998;46(3):305–11.
Brittain HG. Analytical profile of drug substances and excipients. Academic press, editor. California; 1994.
Singh BK, Parwate DV, Shukla SK. Rapid color test identification system for screening of counterfeit fluoroquinolone. E-J Chem. 2009;6:377–84.
Ahmad I, Bano R, Sheraz MA, Ahmed S, Mirza T, Ansari SA. Photodegradation of levofloxacin in aqueous and organic solvents: a kinetic study. Acta Pharm Short Commun. 2013;63:223–9.
UNITED STATES PHARMACOPEIA. National Formulary USP40- NF35. Levofloxacin. USP40-NF35 40ed. ed. Rockville: United States Pharmacopeial Convention; 2017.
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA. Mercury CSD 2.0 new features for the visualization and investigation of crystal structures. J Appl Crystal. 2008;41(2):466–70. https://doi.org/10.1107/S0021889807067908.
Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The cambridge structural database. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2016;72:171–9.
Kugel A, Chisholm B, Ebert S, Jepperson M, Jarabek L, Stafslien S. Antimicrobial polysiloxane polymers and coatings containing pendant levofloxacin. Polym Chem. 2010;1:442–52.
Sagdinc S, Bayari S. Theoretical study of ofloxacin: geometrical parameters and vibrational wavenumbers. J Mol Struct (Theochem). 2004;668:93–9.
Zupančič M, Turel I, Bukovec P, White AJ, Williams DJ. 2001 Synthesis and Characterization of Two Novel Zinc (II) Complexes with Ciprofloxacin. Crystal Structure of [C 17 H 19 N 3 O 3 F] 2⋅[ZnCl 4]⋅ 2H 2 O. Croatica Chemica Acta. 74(1):61-74.
Sahoo S, Kanti Chakraborti C, Mishra C, Nanda N, Naik S. FTIR and XRD investigations of some fluoroquinolones. Int J Pharm Pharm Sci. 2011;3:165–70.
Drugbank. Levofloxacin DB01137 (APRD00477, DB06085) and Levofloxacin hemihydrate DBSALT001001. https://www.drugbank.ca. 2020.
Michot JM, Seral C, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob Agents Chemother. 2005;49:2429–37.
Jiménez-Lozano E, Marqués I, Barrón D, Beltrán JL, Barbosa J. Determination of pKa values of quinolones from mobility and spectroscopic data obtained by capillary electrophoresis and a diode array detector. Anal Chim Acta. 2002;464(1):37–45.
Montero MT, Saiz D, Sitges R, Vázquez JL, Hernández BJ. Influence of physicochemical properties of fluoroquinolones on encapsulation efficiency in liposomes. Int JPharm. 1996;138:113–20.
Lee D-S, Han H-J, Kim K, Park W-B, Cho J-K, Kim J-H. Dissociation and complexation of fluoroquinolone analogues. J Pharm Biomed Anal. 1994;12:157–64.
Takács-Novák K, Noszál B, Hermecz I, Keresztúri G, Podányi B, Szasz G. Protonation equilibria of quinolone antibacterials. J Pharm Sci. 1990;79(11):1023–8.
Herbstein FH. Crystalline Molecular Complexes and Compounds: Structures and Principles. Oxford University Press. 2005;
Santos OM, Freitas JT, Cazedey EC, Araújo MB, Doriguetto AC. Structure, solubility and stability of orbifloxacin crystal forms: Hemihydrate versus anhydrate. Molecules. 2016;21(3):328.
Freitas JTJ, Viana OMMS, Bonfilio R, Ruela ALM, Trevisan MG, Araújo MB. Using thermal analysis as quality control for famotidine polymorph contamination. J Therm Anal Calorim. 2022;147:13405–12.
Acknowledgements
This work received financial support and fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (308893/2019-0 and 312433/2023-9), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) (APQ-01835-18, RED-00116-23, APQ-00544-23 and APQ-05218-23), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (post-doctoral (C.C.M.) and post-graduate (J.T.J.F. and M.B.) grants – Finance Code 001), and PRPPG-UNIFAL-MG (RI grant). We also thanks the facilities for X-ray diffraction, thermal and spectroscopic analysis at UNIFAL-MG.
Author information
Authors and Affiliations
Contributions
Jennifer T. J. Freitas involved in conceptualization, methodology, synthesis, characterization, and writing – original draft, review and editing; Olimpia M. M. S. Viana involved in equilibrium solubility assays and writing – original draft; Cristiane C. de Melo involved in supervision, writing – original draft and review; Monalisa Bitencourt involved in HPLC quantification assays and writing – original draft; Magali B. de Araújo involved in supervision and funding acquisition; Antônio Carlos Doriguetto involved in preparation of the published work, project administration, supervision, formal analysis and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Freitas, J.T.J., Viana, O.M.M.S., de Melo, C.C. et al. Phase transition and solubility of levofloxacin crystal forms: anhydrates versus hydrates. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13252-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13252-y