Abstract
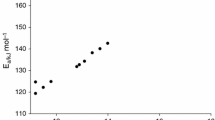
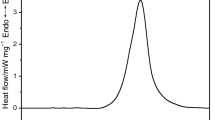
This paper is focused on the effect of hydrogen peroxide (H2O2) on the thermal decomposition of N–methylmorpholine–N–oxide (NMMO). First, accelerating rate calorimetry (ARC) was used to investigate the thermal characteristics of NMMO/H2O2 with different molar ratios. It was found that “onset” temperature was reduced to the minimum temperature with 391.67 K as the molar ratio of NMMO/H2O2 was 3:1. Second, the main decomposed products of NMMO and NMMO/H2O2 were 4–methylmorpholine (NMM) and morpholine (M) identified by liquid chromatography-mass spectrometry (LC–MS). The presence of H2O2 was beneficial to generate more M. Finally, microreactor calorimetry (C80) was employed to calculate the time to maximum rate (TMRad) and the temperature as TMRad is 24 h combined with Friedman and Flynn–Wall–Ozawa models during the decomposition of NMMO and NMMO/H2O2 (molar ratio of 3:1). The apparent activation energy (Ea,ARC) of pure NMMO with 274.99 kJ mol−1 was higher than that of NMMO in the mixture (NMMOmix) with 123.71 kJ mol−1. Besides, TMRad,s of NMMOmix decreased from 39.77 to 27.96 min, and no obvious decrease of TD24 was found compared with the pure NMMO. The results indicated that the presence of H2O2 increased the risk of thermal runaway of NMMOmix, and the mixture had the worst thermal stability as the molar ratio of NMMO/H2O2 was 3:1. It was worthy of mentioning that the most hazardous moment was not the dosing end, but the generated NMMO and H2O2 was reached to 3:1 in the synthesis of NMMO.











Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Jadhav S, Lidhure A, Thakre S, Ganvir V. Modified Lyocell process to improve dissolution of cellulosic pulp and pulp blends in NMMO solvent. Cellulose. 2020;28:973–90. https://doi.org/10.1007/s10570-020-03580-1.
Protz R, Lehmann A, Ganster J, Fink HP. Solubility and spinnability of cellulose-lignin blends in aqueous NMMO. Carbohydr Polym. 2020;251:117027. https://doi.org/10.1016/j.carbpol.2020.117027.
Meng YB, Gao DM, Zhao Q. The synthesis study of N-methyl morpholine oxide. Fine Chem. 1996;03:15–7.
Gao JC, Shi CG, Liu SS, Wang SN, Wang W. Thermal hazards evaluation of the synthesis of N-methylmorpholine-N-oxide. J Therm Anal Calorim. 2022;147(13):7539–49. https://doi.org/10.1007/s10973-021-11052-2.
Rosenau T, Potthast A, Kosma P, Chen CL, Gratzl JS. Autocatalytic decomposition of N-methylmorpholine-N-oxide induced by Mannich intermediates. J Org Chem. 1999;64(7):2166–7. https://doi.org/10.1021/jo982350y.
Rosenau T, French AD. N-methylmorpholine-N-oxide (NMMO): hazards in practice and pitfalls in theory. Cellulose. 2021;28(10):5985–90. https://doi.org/10.1007/s10570-021-03860-4.
Rosenau T, Potthast A, Adorjan I, Hofinger A, Sixta H, Firgo H, Kosma P. Cellulose solutions in N-methylmorpholine-N-oxide (NMMO)–degradation processes and stabilizers. Cellulose. 2002;9(3):283–91. https://doi.org/10.1023/A:1021127423041.
Rosenau T, Schmid P, Potthast A, Kosma P. Stabilization of cellulose solutions in N-methylmorpholine-N-oxide (Lyocell dopes) by addition of an N-oxide as sacrificial substrate. Holzforschung: Int J Biol Chem Phys Technol Wood. 2005;59(5):503–6. https://doi.org/10.1515/HF.2005.083.
Rosenau T, Potthast A, Sixta H, Kosma P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog Polym Sci. 2001;26(9):1763–837. https://doi.org/10.1016/s0079-6700(01)00023-5.
Rosenau T, Potthast A, Milacher W, Adorjan I, Hofinger A, Kosma P. Discoloration of cellulose solutions in N-methylmorpholine-N-oxide (Lyocell) Part 2: isolation and identification of chromophores. Cellulose. 2005;12(2):197–208. https://doi.org/10.1007/s10570-004-0210-3.
Adorjan I, Potthast A, Rosenau T, Sixta H, Kosma P. Discoloration of cellulose solutions in N-methylmorpholine-N-oxide (Lyocell) Part 1: studies on model compounds and pulps. Cellulose. 2005;12(1):51–7. https://doi.org/10.1007/s10570-004-0212-1.
Böhmdorfer S, Hosoya T, Röder T, Potthast A, Rosenau T. A cautionary note on thermal runaway reactions in mixtures of 1-alkyl-3-methylimidazolium ionic liquids and N-methylmorpholine-N-oxide. Cellulose. 2017;24(5):1927–32. https://doi.org/10.1007/s10570-017-1257-2.
Jusner P, Aoki M, Potthast A, Rosenau T. A cautionary note on “exothermic events” upon contact of carbodiimide coupling agents and the cellulose solvent N-methylmorpholine-N-oxide. Cellulose. 2020;27(13):7349–59. https://doi.org/10.1007/s10570-020-03293-5.
Chen K, Lin C, Shu C, Kao C. An evaluation on thermokinetic parameters for hydrogen peroxide at various concentrations by DSC. J Therm Anal Calorim. 2006;85(1):87–9. https://doi.org/10.1007/s10973-005-7362-8.
Lin YJ, Lin ZS, Wang YW. Thermal runaway evaluation using DSC1, VSP2, and kinetics models on Cu etchant and its waste in high-tech etching process. J Therm Anal Calorim. 2021;144(2):285–94. https://doi.org/10.1007/s10973-020-10094-2.
Jiang W, Ni L, Jiang J, Chen Q, Chen Z, Ye S. Thermal hazard and reaction mechanism of the preparation of adipic acid through the oxidation with hydrogen peroxide. AIChE J. 2021;67(1):e17089. https://doi.org/10.1002/aic.17089.
Sun Y, Ni L, Papadaki M, Jiao Z, Zhu W, Jiang JC, Mashuga C, Mannan MS, Wilhite B. Reaction hazard and mechanism study of H2O2 oxidation of 2-butanol to methyl ethyl ketone using DSC, Phi-TEC II and GC-MS. J Loss Prev Process Ind. 2020;66:104177. https://doi.org/10.1016/j.jlp.2020.104177.
Wu D, Qian X, Liu L, Zang N. Effect of inorganic salt and organic acid on the thermal runaway of hydrogen peroxide. J Loss Prev Process Ind. 2019;57:34–40. https://doi.org/10.1016/j.jlp.2018.11.010.
Chi JH, Wu SH, Charpentier JC, Yet-Pole I, Shu CM. Thermal hazard accident investigation of hydrogen peroxide mixing with propanone employing calorimetric approaches. J Loss Prev Process Ind. 2012;25(1):142–7. https://doi.org/10.1016/j.jlp.2011.07.008.
Papadaki M, Gao J. Kinetic models of complex reaction systems. Comput Chem Eng. 2005;29(11–12):2449–60. https://doi.org/10.1016/j.compchemeng.2005.05.021.
Sun Y, Ni L, Papadaki M, Zhu W, Jiang JC, Mashuga C, Wilhite B, Mannan MS. Process hazard evaluation for catalytic oxidation of 2-octanol with hydrogen peroxide using calorimetry techniques. Chem Eng J. 2019;378:122018. https://doi.org/10.1016/j.cej.2019.122018.
Wang WJ, Fang JL, Pan XH, Hua M, Jiang JJ, Ni L, Jiang JC. Thermal research on the uncontrolled behavior of styrene bulk polymerization. J Loss Prev Process Ind. 2019;57:239–44. https://doi.org/10.1016/j.jlp.2018.11.020.
Sun JH, Li XR, Hasegawa K, Liao G. Thermal hazard evaluation of complex reactive substance using calorimeters and Dewar vessel. J Therm Anal Calorim. 2004;76(3):883–93. https://doi.org/10.1023/B:JTAN.0000032272.60526.7c.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19. https://doi.org/10.1016/j.tca.2011.03.034.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci, Part C: Polym Symp. 1964;6(1):183–95. https://doi.org/10.1002/polc.5070060121.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand Sect A Phys Chem. 1966;70A(6):487–523. https://doi.org/10.6028/jres.070A.043.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6. https://doi.org/10.1246/bcsj.38.1881.
Cao CR, Liu SH. Thermal hazard characteristic evaluation of two low-temperature-reactive azo compounds under adiabatic process conditions. Process Saf Environ Prot. 2019;130:231–7. https://doi.org/10.1016/j.psep.2019.08.020.
Sun DX, Miao X, **e CX, Gu J, Li R. Study on thermal properties and kinetics of benzoyl peroxide by ARC and C80 methods. J Therm Anal Calorim. 2012;107(3):943–8. https://doi.org/10.1007/s10973-011-1536-3.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37(1):1–30. https://doi.org/10.1016/0040-6031(80)85001-5.
Pastré J, Wörsdörfer U, Keller A, Hungerbühler K. Comparison of different methods for estimating TMRad from dynamic DSC measurements with ADT 24 values obtained from adiabatic Dewar experiments. J Loss Prev Process Ind. 2000;13(1):7–17. https://doi.org/10.1016/S0950-4230(99)00061-3.
Zhao KJ, Du ZX. Determination of N-methylmorpholine-N-oxide and its degradation products by ultra-performance liquid chromatography-tandem mass spectrometry. J Anal Meas. 2009;28(06):666–70. https://doi.org/10.1159/000210413.
Taeger E, Franz H, Mertel H, Schleicher H, Lang H, Lukanoff B. Probleme der schwefelkohlenstofffreien Verformung von Zellulose zu textilen Zellulosefäden mittels N-methylmorpholine-N-oxids. Formeln Fasern Fertigware. 1985;4:14–22.
Li Y, Liu Y, Chen L, Wu W, Zhao X, Chen W. Research on the thermal hazard of N-Nitrodihydroxyethyl dinitrate (DINA) under the action of diethanolamine. E3S Web of Conferences. EDP Sciences, 2021; 245:03026. https://doi.org/10.1051/e3sconf/202124503026.
Cong YB, Wei ZY, Ma XH, Li ZL, Li QG, Ming X, Cheng CS. Determination of SADT and TMRad of 3-bromo-1-(3, 5-dichloropyridin-2-yl)-4,5-dihydro-1H-pyrazole-5-carboxylic acid: Applying thermal decomposition kinetics. Results Chem. 2021;3:100112. https://doi.org/10.1016/j.rechem.2021.100112.
Musuc AM, Birzan L, Cristea M, Razus D, Razus AC, Oancea D. A DSC study of new compounds based on (E)-3-(azulen-1-yldiazenyl)-1,2,5-oxadiazole. J Therm Anal Calorim. 2021;146(4):1763–72. https://doi.org/10.1007/s10973-020-10164-5.
Lv JY, Chen WH, Chen LP, Tian Y, Yan J. Thermal risk evaluation on decomposition processes for four organic peroxides. Thermochim Acta. 2014;589:11–8. https://doi.org/10.1016/j.tca.2014.05.013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, J., Li, Y., Wang, W. et al. Effect of hydrogen peroxide on the thermal characteristics in the decomposition of N–methylmorpholine–N–oxide. J Therm Anal Calorim 147, 13781–13792 (2022). https://doi.org/10.1007/s10973-022-11680-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11680-2