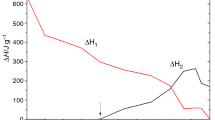
Abstract
In the current work, the thermal curing process of maleinated acrylated epoxidized linseed oil (MaAELO) mixed with reactive diluent, initiator, and accelerator was studied by using isothermal differential scanning calorimetry (DSC) and dynamic rheology. The investigations encompassed (1) the determination of the whole set of apparent kinetic parameters, Ea(α), A(α), and f(α) by using two accurate isoconversional kinetic analysis methods and the compensation effect, (2) the prediction of cure conversion curves, α(t), at arbitrary processing temperatures with all three kinetic parameters in comparison with the prediction based on Ea(α) alone, and (3) the determination of molecular and macroscopic gelation during the thermal cure of MaAELO resin mixture. The thermal cure of MaAELO resin mixture did not start immediately but after a remarkable induction period. It was possible to determine the kinetic parameters and use them to predict the induction period for arbitrary process temperature. In addition, the glass transition temperature, Tg, of thermally cured MaAELO resin mixture was measured by thermomechanical analysis and dynamic DSC.
















Similar content being viewed by others
References
Raquez JM, Deléglise M, Lacrampe MF, Krawczak P. Thermosetting (bio)materials derived from renewable resources: a critical review. Prog Polym Sci. 2010;35:487–509.
Ma S, Li T, Liu X, Zhu J. Research progress on bio-based thermosetting resins. Polym Int. 2016;65:164–73.
Li Q, Ma S, Xu X, Zhu J. Bio-based unsaturated polyesters. In: Thomas S, Hosur M, Chirayil CJ, editors. Unsaturated polyester resins. fundamentals, design, fabrication, and applications. Amsterdam: Elsevier; 2019.
Lu J, Wool RP. Development of new SMC resins and nanocomposites from plant oils. In: Proceedings of the 4th annual SPE automotive composites conference, Troy; 2004.
Lu J, Khot S, Wool RP. New sheet molding compound resins from soybean oil I, Synthesis and characterization. Polym. 2005;46:71–80.
Lu J, Wool RP. Novel thermosetting resins for SMC applications from linseed oil: synthesis, characterization, and properties. J Appl Polym Sci. 2006;99:2481–8.
Lu J, Wool RP. Sheet molding compound resins from soybean oil: thickening behavior and mechanical properties. Polym Eng Sci. 2007;47:1469–79.
Fahimian M, Adhikari D, Raghavan J, Wool RP. Biocomposites from canola oil based resins and hemp and flax fibers. In: 13th European conference on composite materials 2008 Stockholm, Sweden. ECCM13; 2013.
Krämer H. Polyester resins, unsaturated. Ullmann’s encyclopedia of industrial chemistry, vol. 28. Weinheim: Wiley; 2012, p. 613–622.
Zhang Y, Li Y, Wang L, Gao Z, Kessler MR. Synthesis and characterization of methacrylated eugenol as a sustainable reactive diluent for a maleinated acrylated epoxidized soybean oil resin. ACS Sustain Chem Eng. 2017;5:8876–83.
Zhang Y, Li Y, Thakur VK, Wang L, Gu J, Gao Z, Fan B. Wu Q, Kessler MR. Bio-based reactive diluents as sustainable replacements for styrene in MAESO resin. RSC Adv. 2018;8:13780–13788.
Zhang Y, Thakur VK, Li Y, Garrison TF, Gao Z, Kessler MR. Soybean oil-based thermosetting resins with methacrylated vanillyl alcohol as bio-based reactive diluent. Macromol Mater Eng. 2018. https://doi.org/10.1002/mame.201700278.
Zuijderduin R, Böttger W. Cobalt-free curing taking off. Reinf Plast. 2013;57(1):29–32.
Cuadrado TR, Borrajo J, Williams RJJ, Clara FM. On the curing kinetics of unsaturated polyesters with styrene. J Appl Polym Sci. 1983;28(2):485–99.
Avella M, Martuscelli E, Mazzola M. Kinetic study of the cure reaction of unsaturated polyester resins. J Therm Anal. 1985;30:1359–66.
Shin SH, Suh MH, Lee SH, Yie JE, Lee JW. A study on curing kinetics of unsaturated polyester resin. Korean Chem Eng Res. 1987;25(5):522–7.
Ng H, Manas-Zloczower I. A nonisothermal differential scanning calorimetry study of the curing kinetics of an unsaturated polyester system. Polym Eng Sci. 1989;29(16):1097–102.
Ramis X, Salla JM. Effect of the inhibitor on the curing of an unsaturated polyester resin. Polym. 1995;36(18):3511–21.
Salla JM, Ramis X. Comparative study of the cure kinetics of an unsaturated polyester resin using different procedures. Polym Eng Sci. 1996;36(6):835–51.
Zetterlund PB, Johnson AF. A new method for the determination of the Arrhenius constants for the cure process of unsaturated polyester resins based on a mechanistic model. Thermochim Acta. 1996;289:209–21.
Yun YM, Lee SJ, Lee KJ, Lee YK, Nam JD. Composite cure kinetic analysis of unsaturated polyester free radical polymerisation. J Polym Sci, Part B: Polym Phys. 1997;35:2447–56.
Martin JL, Cadenato A, Salla JM. Comparative studies on the non-isothermal DSC curing kinetics of an unsaturated polyester resin using free radicals and empirical models. Thermochim Acta. 1997;306:115–26.
Yousefi A, Lafleur PG, Gauvin R. The effects of cobalt promoter and glass fibres on the curing behaviour of unsaturated polyester resin. J Vinyl Addit Technol. 1997;3(2):157–69.
Yousefi A, Lafleur PG, Gauvin R. Kinetic studies of thermoset cure reactions: a review. Polym Compos. 1997;18(2):157–68.
Lu MG, Shim MJ, Kim SW. Curing behavior of an unsaturated polyester system analyzed by Avrami equation. Thermochim Acta. 1998;323:37–42.
Ramis X, Salla JM. Effect of the initiator content and temperature on the curing of an unsaturated polyester resin. J Polym Sci, Part B: Polym Phys. 1999;37:751–68.
Salla JM, Cadenato A, Ramis X, Morancho JM. Thermoset cure kinetics by isoconversional methods. J Therm Anal Calorim. 1999;56:771–81.
Vilas JL, Laza JM, Garay MT, Rodriguez M, Leon LM. Unsaturated polyester resins cure: Kinetic, rheologic, and mechanical-dynamical analysis. I. Cure Kinetics by DSC and TSR. J Appl Polym Sci. 2001;79:447–457.
Cook WD, Lau M, Mehrabi M, Dean K, Zipper M. Control of gel time and exotherm behaviour during cure of unsaturated polyester resins. Polym Int. 2001;50:129–34.
Rouison D, Sain M, Couturier M. Resin transfer moulding of natural fibre reinforced plastic. I. Kinetic study of an unsaturated polyester resin containing an inhibitor and various promoters. J Appl Polym Sci. 2003;89:2553–2561.
Rouison D, Sain M, Couturier M. Resin transfer molding of natural fibre reinforced composites: cure simulation. Compos Sci Technol. 2004;64:629–44.
Boyard N, Sinturel C, Vayer M, Erre R. Morphology and cure kinetics of unsaturated polyester resin/block copolymer blends. J Appl Polym Sci. 2006;102:149–65.
Worzakowska M. Kinetics of the curing reaction of unsaturated polyester resins catalyzed with new initiators and a promoter. J Appl Polym Sci. 2006;102:1870–6.
Martin JL. Kinetic analysis of two DSC peaks in the curing of an unsaturated polyester resin catalysed with methylethylketone peroxide and cobalt octoate. Polym Eng Sci. 2007;47(1):62–70.
Gao J, Dong C, Du Y. Nonisothermal curing kinetics and physical properties of unsaturated polyester modified with EA-POSS. Int J Polym Mater. 2010;59:1–14.
Janković B. The kinetic analysis of isothermal curing reaction of an unsaturated polyester resin: Estimation of the density distribution function of the apparent activation energy. Chem Eng J. 2010;162:331–40.
Kuppusamy RRP, Neogi S. Influence of curing agents on gelation and exotherm behaviour of an unsaturated polyester resin. Bull Mater Sci. 2013;36(7):1217–24.
Poorabdollah M, Beheshty MH, Atai M, Vafayan M. Cure kinetic study of organoclay-unsaturated polyester resin nanocomposites by using advanced isoconversional approach. Polym Compos. 2013;34(11):1824–31.
Aktas A, Krishnan L, Kandola B, Boyd SW, Shenoi RA. A cure modelling study of an unsaturated polyester resin system for the simulation of curing of fibre-reinforced composites during the vacuum infusion process. J Compos Mater. 2014;49(20):2529–40.
S Subaşı S, Poyraz B, Eren Ş, Gökçe N, Aykanat B. Initiator effect on chemical, thermal and mechanical Properties of polyester based composites. In: Proceedings of the 2nd international conference on civil and environmental engineering, Cappadocia, Nevşehir, 2017.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. Review: ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. Springer, New York; 2015.
Sbirrazzuoli N. Determination of pre-exponential factors and of the mathematical functions f(α) or G(α) that describe the reaction mechanism in a model-free way. Thermochim Acta. 2013;564:59–69.
Roduit B, Folly P, Berger B, Mathieu J, Sarbach A, Andres H, Ramin M, Vogelsanger B. Evaluating sadt by advanced kinetics-based simulation approach. J Therm Anal Calorim. 2008;93(1):153–61.
Winter HH. Can the gel point of as cross-linking polymer be detected by the G′–G′′ crossover? Polym Eng Sci. 1987;27(22):1698–702.
Sbirrazzuoli N, Mititelu-Mija A, Vincent L, Alzina C. Isoconversional kinetic analysis of stoichometric and off-stoichometric epoxy-amine cures. Thermochim Acta. 2006;447:167–77.
Alonso MV, Oliet M, García J, Rodríguez F, Echeverría J. Gelation and isoconversional kinetic analysis of lignin-phenol-formaldehyde resol resins cure. Chem Eng J. 2006;122:159–66.
Galwey AK, Brown ME. Kinetic background to thermal analysis and calorimetry. In: Brown ME (Ed.). Handbook of thermal analysis and calorimetry, volume 1: principles and practice. Amsterdam: Elsevier; 1998.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178–83.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry: application to a phenolic plastic. J Polym Sci. 1964;C6:183–195.
Flynn JH. A general differential technique for the determination of parameters for d(α)/dt = f(α) A exp(-E/RT). J Therm Anal. 1991;37(2):293–305.
Galwey AK, Brown ME. Application of the Arrhenius equation to solid state kinetic: can this be justified? Thermochim Acta. 2002;386:91–8.
Vyazovkin S. Model-free kinetics. Staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83(1):45-51.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Kim SY, Choir DG, Yang SM. Rheological Analysis of the gelation behavior of tetraethylorthosilane/vinyltriethoxysilane hybrid solutions. Korean J Chem Eng. 2002;19(1):190–6.
ASTM E1545-11 (2016). Standard test method for assignment of the glass transition temperature by thermomechanical analysis.
Kamal MR. Thermoset characterization for moldability analysis. Polym Eng Sci. 1974;14:231–9.
Sourour S, Kamal MR. Differential scanning calorimetry of epoxy cure: Isothermal cure kinetics. Thermochim Acta. 1976;14:41–59.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int Rev Phys Chem. 1998;17(3):407–33.
Sbirrazzuoli N, Vyazovkin S, Mititelu A, Sladic C, Vincent L. A study of epoxy-amine cure kinetics by combining isoconversional analysis with temperature modulated DSC and dynamic rheometry. Macromol Chem Phys. 2003;204(15):1815–21.
Acknowledgements
This scientific work was funded by the Austrian Ministry for Transport, Innovation and Technology in frame of the program “Produktion der Zukunft” under Contract No. 858688.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wuzella, G., Mahendran, A.R., Beuc, C. et al. Isoconversional cure kinetics of a novel thermosetting resin based on linseed oil. J Therm Anal Calorim 142, 1055–1071 (2020). https://doi.org/10.1007/s10973-020-09529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09529-7