Abstract
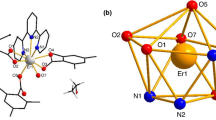
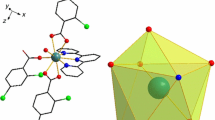
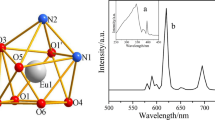
Two lanthanide complexes [La(3,4-DMBA)3]n (1) and [Tb(3,4-DMBA)3(DIPY)2]2 (2) (where 3,4-DMBA = 3,4-dimethylbenzoate, DIPY = 2,2′-bipyridine) have been synthesized and then characterized. The single crystals of complexes 1 and 2 were obtained. Complex 1 adopts a distorted monocapped square antiprismatic molecular geometry, while complex 2 forms a distorted square antiprism. Complex 1 is stitched together by edge sharing through carboxylate ligands to form 1D chains along the b axis. The structure of complex 2 consists of 1D chains along the c axis by two kinds of π···π stacking interactions. Two complexes were characterized by elemental analysis, powder X-ray diffraction and IR spectra. The complex 2 shows typical green luminescence in the solid state. Thermal decomposition mechanisms of complexes were discussed by TG–DTG techniques. In the temperature range from 257.15 to 413.15 K, the molar heat capacities of the complexes were measured by DSC. The smoothed heat capacities and thermodynamic functions of complexes were calculated on the basis of fitted polynomial and thermodynamic equations.







Similar content being viewed by others
References
Ye JW, Wang QQ, Gao HZ, Lu XY, Gong WT, Lin Y, Ning GL. Effect of auxiliary-ligand on assembly of lanthanide(III) complexes with quinoline-2-carboxylic acid: synthesis, structure, photoluminescent and magnetic properties. Inorg Chim Acta. 2012;384:1–7.
Li M, Lan Y, Ako AM, Wernsdorfer W, Anson CE, Buth G, Powell AK, Wang Z, Gao S. A family of 3d-4f octa-nuclear [MnIII 4LnIII 4] wheels (Ln = Sm, Gd, Tb, Dy, Ho, Er, and Y): synthesis, structure, and magnetism. Inorg Chem. 2010;49:11587–94.
Wang Y, ** CW, He SM, Ren N, Zhang JJ. Five novel lanthanide complexes with 2-chloroquinoline-4-carboxylic acid and 1,10-phenanthroline: Crystal structures, molecular spectra, thermal properties and bacteriostatic activities. J Mol Struct. 2016;1125:383–90.
Zhang S, Yang Y, **a ZQ, Liu XY, Yang Q, Wei Q, **e G, Chen SP, Gao SL. Eu-MOFs with 2-(4-carboxyphenyl)imidazo[4,5-f]-1,10-phenanthroline and ditopic carboxylates as coligands: synthesis, structure, high thermostability, and luminescence properties. Inorg Chem. 2014;53:10952–63.
Sharma G, Narula AK. Synthesis of Eu(III) complexes with 2-aminopyridine and 1,10-phenanthroline: structural, optical, thermal and morphological studies. Sens. Actuators B: Chem. 2015;215:584–91.
Misra SN, Gagnani MA, Indira DM, Shukla RS. Biological and clinical aspects of lanthanide coordination compounds. Bioinorg Chem Appl. 2004;2:155–92.
Cao HY, Liu QY, Gao MJ, Wang YL, Chen LL, Liu Y. Ionothermal syntheses, crystal structures and luminescence of three three-dimensional lanthanide-1,4-benzenedicarboxylate frameworks. Inorg Chim Acta. 2014;414:226–33.
Decadt R, Van Hecke K, Depla D, Leus K, Weinberger D, Van Driessche I, Van Der Voort P, Van Deun R. Synthesis, crystal structures, and luminescence properties of carboxylate based rare-earth coordination polymers. Inorg Chem. 2012;51:11623.
Liu TF, Zhang W, Sun WH, Cao R. Conjugated ligands modulated sandwich structures and luminescence properties of lanthanide metal-organic frameworks. Inorg Chem. 2011;50:5242–48.
Stan CS, Rosca I, Sutiman D, Secula MS. Highly luminescent europium and terbium complexes based on succinimide and N-hydroxysuccinimide. J Rare Earths. 2012;30:401–7.
Listkowski A, Osińska W, Mohanraj J, Pietraszkiewicz M, Dutkiewicz G, Borowiak T. Synthesis and photoluminescence properties of novel lanthanide complexes based on pyrazolone Schiff bases. Synth Met. 2012;162:1285–91.
Ay B, Yildiz E, Kani İ. Novel heteroleptic lanthanide organic frameworks containing pyridine-2,5-dicarboxylic acid and in situ generated piperazine-2,5-dicarboxylic acid from piperazine: Hydrothermal synthesis and luminescent properties. J Solid State Chem. 2016;233:44–51.
Wang Y, Zhao QQ, Ren N, Zhang JJ, Geng LN, Wang SP. Crystal structures, thermal properties, and luminescent properties of two novel mononuclear lanthanide complexes with 2,4-dichlorobenzoic acid and 2,2′:6′,2″-terpyridine. J Therm Anal Calorim. 2016;126:1703–12.
Song XQ, Zheng JR, Liu WS, Ju ZH. Synthesis, structure and spectroscopic properties of rare earth complexes with a new aryl amide 2,2′-bipyridine derivative. Spectrochim Acta A Mol Biomol Spectrosc. 2008;69:49–55.
Marques LF, Cuin A, de Carvalho GSG, dos Santos MV, Ribeiro SJL, Machado FC. Energy transfer process in highly photoluminescent binuclear hydrocinnamate of europium, terbium and gadolinium containing 1,10-phenanthroline as ancillary ligand. Inorg Chim Acta. 2016;441:67–77.
Li HF, Yan PF, Chen P, Wang Y, Xu H, Li GM. Highly luminescent bis-diketone lanthanide complexes with triple-stranded dinuclear structure. Dalton Trans. 2012;41:900–7.
Wang Y, Song Y, Pan ZR, Shen YZ, Hu Z, Guo ZJ, Zheng HG. Unprecedented NaI–CuII–LnIII heterometallic coordination polymers based on 3,5-pyrazoledicarboxylate with both infinite cationic and anionic chains. Dalton Trans. 2008;41:5588–92.
You LX, Wang SJ, **ong G, Ding F, Meert KW, Poelman D, Smet PF, Ren BY, Tian YW, Sun YG. Synthesis, structure and properties of 2D lanthanide coordination polymers based on N-heterocyclic arylpolycarboxylate ligands. Dalton Trans. 2014;43:17385–94.
Marques LF, Cantaruti AAB, Correa CC, Lahoud MG, da Silva RR, Ribeiro SJL, Machado FC. First crystal structures of lanthanide-hydrocinnamate complexes: hydrothermal synthesis and photophysical studies. J Photoch Photobio A: Chem. 2013;252:69–76.
Feng SY, Li WX, Guo F, Cao XF. Luminescence enhancement of terbium(III) perchlorate by 2,2′-dipyridyl on bis(benzylsulfinyl)methane complex and luminescence mechanism. Luminescence. 2014;29:791–97.
Esmonde-White K. Raman spectroscopy of soft musculoskeletal tissues. Appl Spectrosc. 2014;68:1203–18.
Sun SJ, Wang JF, Ren N, Zhang JJ, Ye HM, Wang SP. Crystal structures, luminescent properties and thermal decomposition kinetics of some binuclear lanthanide complexes with 2,3-dichlorobenzoic acid anion and 2,2′-bipyridine. Struct Chem. 2011;23:79–89.
Qi XX, Wu JC, Ren N, Zhao CL, Zhang JJ, Zong GC, Gao J. Novel lanthanide complexes constructed from 3, 4-dimethoxybenzoic acid: crystal structures, spectrum and thermochemical properties. Thermochim Acta. 2015;615:1–7.
Zhou JM, Shi W, Xu N, Cheng P. Highly selective luminescent sensing of fluoride and organic small-molecule pollutants based on novel lanthanide metal-organic frameworks. Inorg Chem. 2013;52:8082–90.
Deng ZP, Kang W, Huo LH, Zhao H, Gao S. Rare-earth organic frameworks involving three types of architecture tuned by the lanthanide contraction effect: hydrothermal syntheses, structures and luminescence. Dalton Trans. 2010;39:6276–84.
Zong GC, Huo JX, Ren N, Zhang JJ, Qi XX, Gao J, Geng LN, Wang SP, Shi SK. Preparation, characterization and properties of four new trivalent lanthanide complexes constructed using 2-bromine-5-methoxybenzoic acid and 1,10-phenanthroline. Dalton Trans. 2015;44:14877–86.
Carter KP, Zulato CHF, Cahill CL. Exploring supramolecular assembly and luminescent behavior in a series of RE-p-chlorobenzoic acid-1,10-phenanthroline complexes. CrystEngComm. 2014;16:10189–202.
Carter KP, Pope SJA, Cahill CL. A series of Ln-p-chlorobenzoic acid–terpyridine complexes: lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm. 2014;16:1873–84.
** CW, Shen PP, Ren N, Geng LN, Zhang JJ. Structure, luminescent and thermal properties of two novel lanthanide complexes with 3,4-diethoxybenzoic acid and 5,5′-dimethy-2,2′-bipyridine. J Therm Anal Calorim. 2016;126:1549–58.
Liu YQ, Xu YP. Synthesis, structure, and fluorescent study of complex: bis(4,4′-dimethyl-2,2′-bipyridinato)-tris(nitrato-o, o′)-terbium(III). Synth React Inorg M. 2015;46:238–40.
Hou XY, Wang X, Gao LJ, Fu F, Wang JJ, Cao J. Synthesis, structures, luminescence, and magnetic properties of two three-dimensional lanthanide organic frameworks comprising pyrazine-2,3-dicarboxylic acid. Z Anorg Allg Chem. 2014;640:2072–77.
Ilmi R, Iftikhar K. Photophysical properties of Lanthanide(III) 1,1,1-trifluoro -2,4-pentanedione complexes with 2,2′-bipyridyl: an experimental and theoretical investigation. J Photoch Photobio A: Chem. 2017;333:142–55.
Li HN, Li HY, Li LK, Xu L, Hou K, Zang SQ, Mak TCW. Syntheses, structures, and photoluminescent properties of lanthanide coordination polymers based on a zwitterionic aromatic polycarboxylate ligand. Cryst Growth Des. 2015;15:4331–40.
Luo GH, Gao XH, Pan L, Lv XC, Tan ZC. Low-temperature molar heat capacities and thermodynamic properties of a new rare earth complex Er2(μ 2-Gly)6(H2O)4·Na2(ClO4)8(H2O)2·4H2O. J Therm Anal Calorim. 2016;126:871–79.
Lu DF, Di YY, Tan ZC, Dou JM. Low-temperature heat capacities, and thermodynamic properties of solid–solid phase change material bis(1-octylammonium) tetrachlorocuprate. J Therm Anal Calorim. 2012;111:213–18.
Zhang YY, Ren SX, Ren N, Zhang JJ, Geng LN, Wang SP, Shi SK. Crystal structures, spectroscopic, and thermal properties of Dysprosium(III) and Europium(III) complexes with 3-chloro-4-methoxybenzoic and 1,10-phenanthroline. J Therm Anal Calorim. 2014;119:1803–10.
Tang K, Liu HM, Ren N, Zhang JJ, Wu KZ. Crystal structures, luminescence, and thermal properties of lanthanide complexes with 2,3,4-trimethoxybenzoic acid and 1,10-phenanthroline. J Chem Thermodyn. 2012;47:428–36.
Acknowledgements
The research work was supported by the National Natural Science Foundation of China (No. 21473049) and the Natural Science Foundation of Hebei Province (No. B2016205207).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, PP., Ren, N., Zhang, JJ. et al. Crystal structures, luminescent and thermal properties of lanthanide complexes with 3,4-dimethylbenzoic acid and 2,2′-bipyridine. J Therm Anal Calorim 131, 1699–1707 (2018). https://doi.org/10.1007/s10973-017-6643-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6643-3