Abstract
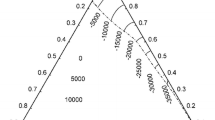
The results of calorimetric investigations of liquid In–Sn alloys using Oelsen calorimetry are presented in this article. Based on obtained enthalpy space diagram and enthalpy isotherm diagram, thermodynamic parameters for the liquid In–Sn alloys, including mixing and excess integral and partial molar quantities at temperature of 600 K, have been determined and compared with available literature data.






Similar content being viewed by others
References
Speckbrock G, Kamitz S, Alt M, Schmitt H. Low melting gallium, indium, and tin eutectic alloys, United States Patent 6019509; 2000.
White CET, Okamoto H, editors. Phase diagrams of indium alloys and their engineering applications. Utica, NY: Indium Corporation of America, Ohio: Materials Information Soc., Materials Park; 1992.
http://www.crct.polymtl.ca/fact/Documentation/BINARY/In-Sn.jpg.
Vicentini M, Paoletti A. Self diffusion in liquid In-Sn alloys. Phys Astron. 1959;14:1373–80.
Swarnalata N, Padmini ARKL. Ultrasonic velocities and elastic constants in tin–indium alloys. Patrmana. 1984;23:155–63.
Maciejewski S. Band structure and fermi surface on In3Sn alloys in the β-phase. Phys Status Solidi B. 2006;66:633–38.
Degtyareva VF. The fcc-bcc bain path in In-Sn and related alloys at ambient and high pressure. J Phys. 2009;21:95702–6.
Terpilowski J, Przedziecka-Mycielska E. EMF measurements of In-Sn alloys. Arch Hutn. 1960;5:281–9.
Cakir O, Alpaut O. Thermodynamic properties of solid In-Sn alloys. J Less-Common Met. 1988;141:11–27.
Kleppa OJ. Calorimetric investigation of the In-Sn system. J Phys Chem. 1956;60:842–50.
Wittig FE, Scheidt P. Direct reaction calorimetry of In-Sn alloys. Z Phys Chem. 1961;28:120–6.
Yazawa A, Kawashima T, Itagaki K. Thermodynamics of the In-Sn system. J Jpn Inst Met Sendai. 1968;32:1281–7.
Brunetti B, Gozzi D, Iervolino M, Piacente V, Zanicchi G, Parodi N, Borzone G. Bismuth activity in lead-free solder Bi-In-Sn-alloys. Calphad. 2006;30:431–42.
Hultgren R, Desai PD, Hawkins DT, Gleiser M, Kelley KK. Selected values of thermodynamic properties of binary alloys. Metals Park, OH: American Society of Metals; 1973.
Dinsdale AT, Watson A, Kroupa A, Zemanova A, Vrestal J, Vizdal J. COST531 thermodynamic database, Ver. 2.2; 2006.
Oelsen W, Zuhlke P. Zur thermodynamischen Analyse VIII. Arch Eisenhuttenwes. 1956;27:743–52.
Oelsen W, Johannsen F, Podgornik A. Kalorimetrie und Thermodynamik der Blei-Antimon-legierungen. Zeitsch Erzbergbau Metallhüttenwes. 1956;9:1–11.
Oelsen W, Schürmann E, Weigt HJ, Oelsen O. Zur thermodynamischen Analyse IV. Arch Eisenhuttenwes. 1956;27:487–511.
Pool MJ, Predel B, Schulheiss E. Application of the Setaram high temperature calorimeter for determination of mixing enthalpies of liquid alloys. Thermochim Acta. 1979;28:349–58.
Zivkovic D, Manasijevic D, Zivković Z. Thermodynamic and phase diagram investigation of Pb-BiIn section in Pb-Bi-In ternary system. Thermochim Acta. 2004;417:119–25.
Sorai M, editors. Comprehensive handbook of calorimetry and thermal analysis. Chichester: Wiley; 2004. p 297.
Zivkovic D, Katayama I, Gomidzelovic L, Manasijevic D, Novakovic R. Comparative thermodynamic study and phase equilibria of the Bi-Ga-Sn ternary system. Int J Mater Res. 2007;98:1025–30.
Gomidzelovic L, Zivkovic D. Thermodynamic analysis of AuIn-Sb system using Oelsen calorimetry and predicting methods. J Therm Anal Calorim. 2009;98:743–8.
Saunders N, Miodownik AP. CALPHAD calculation of phase diagrams—a comprehensive guide. Amsterdam: Elsevier; 1998.
Acknowledgements
The authors are grateful to support of the Ministry of Science and Technological Development of the Republic of Serbia under project No 142043. Presented investigations were done also in the frame of COST project MP0602.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Živković, D., Mitovski, A., Balanović, L. et al. Thermodynamic analysis of liquid In–Sn alloys using Oelsen calorimetry. J Therm Anal Calorim 102, 827–830 (2010). https://doi.org/10.1007/s10973-010-0785-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0785-x