Abstract
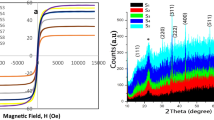
Magnetite nanoparticles (α-Fe3O4) were successfully prepared by a chemical co-precipitation technique. Modification in electrical properties of α-Fe3O4 by Cu2+ dopant for the modification in electrical properties was deliberated. As the Cu2+ dopant content increased from 5 to 10%, the average crystallite size decreased from 2.96 to 2.93 nm. The synthesized sample doped with 5% exhibited the porous nature and least agglomeration. The optical studies revealed that energy band gap increased from 1.76–1.83 eV by enhancing Cu2+ content from 5 to 10%. The electrical studies revealed that the electrical conductivity decreased from 4.04 × 10−5 to 9.17 × 10−6 ℧ cm−1. The obtained consequences revealed that desired properties of Cu+2 doped Fe3O4 NPs can be obtained by controlling the substituting content in host material. The Fe3O4 NPs with Cu2+ do** exhibited higher electrical conductivity and become an excellent candidate for development of electronic and optoelectronic devices, such as, photodetector, sensors and energy storage devices.
Graphical Abstract

Highlights
-
Modification in electrical properties of α-Fe3O4 by Cu2+ dopant for the modification in electrical properties was deliberated.
-
As the Cu2+ dopant content increased from 5 to 10%, the average crystallite size decreased from 2.96 nm to 2.93 nm.
-
The synthesized sample doped with 5% exhibited the porous nature and least agglomeration.
-
The optical studies revealed that energy band gap increased from 1.76 to 1.83 eV by enhancing Cu2+ content from 5 to 10%. The electrical studies revealed that the electrical conductivity decreased from 4.04 × 10−5 to 9.17 × 10−6 ℧ cm−1.
-
The obtained consequences revealed that desired properties of Cu+2 doped Fe3O4 NPs can be obtained by controlling the substituting content in host material.
-
The Fe3O4 NPs with Cu2+ do** exhibited higher electrical conductivity and become an excellent candidate for development of electronic and optoelectronic devices, such as, photo-detector, sensors and energy storage devices.







Similar content being viewed by others
References
Niculescu AG, Chircov C, Grumezescu AM (2022) Magnetite nanoparticles: Synthesis methods–a comparative review. Methods 199:16–27
Chifiriuc MC, Grumezescu AM, Andronescu E, Ficai A, Cotar AI, Grumezescu V, Radulescu R (2013) Water dispersible magnetite nanoparticles influence the efficacy of antibiotics against planktonic and biofilm embedded Enterococcus faecalis cells. Anaerobe 22:14–19
Sebastian Cabeza V (2016) Chapter High and Efficient Production of Nanomaterials by Microfluidic Reactor Approaches
Shaoqiang Z, Dong T, Geng Z, Lin H, Hua Z, Jun H, Wei Z (2021) The influence of grain size on the magnetic properties of Fe3O4 nanocrystals synthesized by solvothermal method. J Sol Gel Sci Technol 98(2):422–429
Mihai AD, Chircov C, Grumezescu AM, Holban AM (2020) Magnetite nanoparticles and essential oils systems for advanced antibacterial therapies. Int J Mol Sci 21(19):7355
Jayakrishnan P, Ramesan MT (2017) Studies on the effect of magnetite nanoparticles on magnetic, mechanical, thermal, temperature dependent electrical resistivity and DC conductivity modeling of poly (vinyl alcohol-co-acrylic acid)/Fe3O4 nanocomposites. Mater Chem Phys 186:513–522
Kumar DS, Naidu KCB, Rafi MM, Nazeer KP, Begam AA, Kumar GR (2018) Structural and dielectric properties of superparamagnetic iron oxide nanoparticles (SPIONs) stabilized by sugar solutions. Mater Sci Pol 36:123–133
Zhao F, Zhang B, Feng L (2012) Preparation and magnetic properties of magnetite nanoparticles. Mater Lett 68:112–114
Afzal F, Rehman AU, Rafique MD, Ansar MT, Munir MA, Hussain Q (2021) High-performance SnO2 nanotubes as efficient electrode materials. Nano Struct Nano Objects 26:100740
Kamboj, N, Dey, A, Lama, P, Majumder, M, Sengupta, S, & Metre, RK (2023). A closed-shell phenalenyl-based dinuclear iron (iii) complex as a robust cathode for a one-compartment H2O2 fuel cell. Dalton Trans 52:17163–17175
Chen GH, Chen HS (2020) Nanometer-thick Sol–Gel Silica–Titania film infused with superparamagnetic Fe3O4 nanoparticles for electromagnetic interference shielding. ACS Appl Nano Mater 3(9):8858–8865
Mohammadi H, Nekobahr E, Akhtari J, Saeedi M, Akbari J, Fathi F (2021) Synthesis and characterization of magnetite nanoparticles by co-precipitation method coated with biocompatible compounds and evaluation of in-vitro cytotoxicity. Toxicol Rep 8:331–336
Căpraru A, Moacă EA, Păcurariu C, Ianoş R, Lazău R, Barbu-Tudoran L (2021) Development and characterization of magnetic iron oxide nanoparticles using microwave for the combustion reaction ignition, as possible candidates for biomedical applications. Powder Technol 394:1026–1038
Hossain MS, Akter Y, Shahjahan M, Bashar MS, Begum MHA, Hossain MM, Al-Mamun M (2019) Influence of Ni substitution on structural, morphological, dielectric, magnetic and optical properties of Cu–Zn ferrite by double sintering sol–gel technique. J Adv Dielectr 9(02):1950020
Parhizkar J, Habibi MH (2017) Synthesis, characterization and photocatalytic properties of Iron oxide nanoparticles synthesized by sol-gel autocombustion with ultrasonic irradiation. Nanochem Res 2(2):166–171
Kamboj N, Betal A, Majumder M, Sahu S, Metre RK (2023) Redox switching behavior in resistive memory device designed using a solution-processable phenalenyl-based Co (II) complex: experimental and DFT studies. Inorg Chem 62(10):4170–4180
Fu R, ** X, Liang J, Zheng W, Zhuang J, Yang W (2011) Preparation of nearly monodispersed Fe3O4/SiO2 composite particles from aggregates of Fe3O4 nanoparticles. J Mater Chem 21(39):15352–15356
Hedayati K, Goodarzi M, Ghanbari D (2017) Hydrothermal synthesis of Fe3O4 nanoparticles and flame resistance magnetic poly styrene nanocomposite. J Nanostruct 7(1):32–39
Jung E, Kim SW, Cho A, Kim YJ, Jeong GJ, Kim J, Yu T (2019) Synthesis of sub 3 nm-sized uniform magnetite nanoparticles using reverse micelle method for biomedical application. Materials 12(23):3850
Prabowo B, Khairunnisa T, Nandiyanto ABD (2018) Economic perspective in the production of magnetite (Fe3O4) nanoparticles by co-precipitation method. World Chem Eng J 2(2):1–4
Takai ZI, Mustafa MK, Asman S, Sekak KA (2019) Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. Int J Nanoelectron Mater 12:37–46
Hu P, Chang T, Chen WJ, Deng J, Li SL, Zuo YG, Volinsky AA (2019) Temperature effects on magnetic properties of Fe3O4 nanoparticles synthesized by the sol-gel explosion-assisted method. J Alloy Compd 773:605–611
Kushwaha P, Chauhan P (2022) Influence of different surfactants on morphological, structural, optical, and magnetic properties of α-Fe2O3 nanoparticles synthesized via co-precipitation method. Appl Phys A 128(1):1–14
Sekar AD, Jayabalan T, Muthukumar H, Chandrasekaran NI, Mohamed SN, Matheswaran M (2019) Enhancing power generation and treatment of dairy waste water in microbial fuel cell using Cu-doped iron oxide nanoparticles decorated anode. Energy 172:173–180
Rani S, Varma GD (2015) Superparamagnetism and metamagnetic transition in Fe3O4 nanoparticles synthesized via co-precipitation method at different pH. Phys B Condens Matter 472:66–77
Chavan VD, Kothavale VP, Sahoo SC, Kollu P, Dongale TD, Patil PS, Patil PB (2019) Adsorption and kinetic behavior of Cu (II) ions from aqueous solution on DMSA functionalized magnetic nanoparticles. Phys B Condens Matter 571:273–279
Shatooti S, Mozaffari M, Reiter G, Zahn D, Dutz S (2022) An investigation on the heat dissipation in Zn-substituted magnetite nanoparticles, coated with citric acid and pluronic F127 for hyperthermia application. Phys B Condens Matter 625:413468
Anandhi JS, Arun T, Joseyphus RJ (2020) Role of magnetic anisotropy on the heating mechanism of Co-doped Fe3O4 nanoparticles. Phys B Condens Matter 598:412429
Mocherla PS, Karthik C, Ubic RNAR, Ramachandra Rao MS, Sudakar C (2013) Tunable bandgap in BiFeO3 nanoparticles: the role of microstrain and oxygen defects. Appl Phys Lett 103(2):022910
Kamboj N, Mali G, Lama P, Erande RD, Metre RK (2022) Designing a redox noninnocent phenalenyl-based copper (II) complex: an autotandem catalyst for the selective oxidation of polycyclic aromatic hydrocarbons (PAHs). ACS Omega 7(10):8789–8797
Munir MA, Naz MY, Shukrullah S, Farooq MU, Kamran K, Irfan M, Ghanim AAJ (2023) Testing of magnetic and dielectric traits of microwave plasma treated NiCuZn spinel ferrites for efficient energy storage and high-frequency applications. Mater Sci Eng B 291:116374
Chandekar KV, Kant KM (2017) Strain induced magnetic anisotropy and 3d7 ions effect in CoFe2O4 nanoplatelets. Superlattices Microstructures 111:610–627
Chandekar KV, Kant KM (2018) Size-strain analysis and elastic properties of CoFe2O4 nanoplatelets by hydrothermal method. J Mol Struct 1154:418–427
Shah ZH, Riaz S, Kayani ZN, Naseem S (2016) Size distribution and magnetic optimization of phase pure chromium doped magnetite nanoparticles. In The 2016 World Congress on Advances in Civil, Environmental and Materials Research (ACEM’16). ICC Jeju, Jeju Island, Korea
Tahir MS, Lee JW, Rabani I, Afzal F, Han YJ, Park HY, Seo YS (2023) Analysis of the porosity of ZIF-8 and ZIF-8@ CNF membranes using positron annihilation lifetime spectroscopy (PALS). J Radioanal Nucl Chem 332:3967–3975.
López YC, Acevedo-Peña P, Ortega GA, Reguera E (2021) Unraveling the Fe3O4 NPs role in self-assembled magnetic zinc oxide nanorods for methylene blue photodegradation. J Photochem Photobiol A Chem 421:113514
Rabani I, Tahir MS, Lee WI, Truong HB, Dastgeer G, Seo YS (2023) Palladium nanoparticle formation on boron nitride nanotubes and their photocatalytic performance with visible light. J Clean Prod 420:138324
Butnoi P, Pangon A, Berger R, Butt HJ, Intasanta V (2021) Electrospun nanocomposite fibers from lignin and iron oxide as supercapacitor material. J Mater Res Technol 12:2153–2167
Badawi A, Althobaiti MG, Alharthi SS, Al-Baradi AM (2021) Tailoring the optical properties of CdO nanostructures via barium do** for optical windows applications. Phys Lett A 411:127553
Chandekar KV, Shkir M, Khan A, Sayed MA, Alotaibi N, Alshahrani T, AlFaify S (2021) Significant and systematic impact of yttrium do** on physical properties of nickel oxide nanoparticles for optoelectronics applications. J Mater Res Technol 15:2584–2600
Chandekar KV, Shkir M, Palanivel B, Ahmad Z, Algarni H, AlFaify S (2021) Comparative study of Pr-doped and undoped PbS nanostructures facilely synthesized for optoelectronic applications. Solid State Sci 122:106773
Shkir M, Chandekar KV, Alshahrani T, Kumar A, Khan A, AlFaify S (2020) An impact of La do** content on key physical properties of PbS spherical nanoparticles facilely synthesized via low temperature chemical route. Eur Phys J 135(10):816
Yousuf MA, Jabeen S, Shahi MN, Khan MA, Shakir I, Warsi MF (2020) Magnetic and electrical properties of yttrium substituted manganese ferrite nanoparticles prepared via micro-emulsion route. Results Phys 16:102973
Adebayo LL, Soleimani H, Guan BH, Öchsner A, Sabet M, Yusuf JY, Ali H (2021) A simple route to prepare Fe3O4@C microspheres as electromagnetic wave absorbing material. J Mater Res Technol 12:1552–1563
Jabir MS, Nayef UM, Kadhim WKA (2019) Polyethylene glycol-functionalized magnetic (Fe3O4) nanoparticles: A novel DNA-mediated antibacterial agent. Nano Biomed Eng 11(1):18–27
Afzal S, Khan R, Zeb T, Ali S, Khan G, Hussain A (2018) Structural, optical, dielectric and magnetic properties of PVP coated magnetite (Fe3O4) nanoparticles. J Mater Sci Mater Electron 29(23):20040–20050
Acknowledgements
This work was funded by the Researchers Supporting Project Number (RSP2023R243) King Saud University, Riyadh, SaudiArabia.
Author contributions
SS: Conceptualization, Writing-Original Draft, Methodology, Investigation, Data Curation, Validation. MHJ: Formal analysis, Writing and editing, AAA: Language Editing, Writing-Review & Editing, MZHBM: Language Editing, Writing-Review & Editing, TY: Writing-Review & Editing, MRA: Writing-Review & Editing, AA: Writing-Review & Editing, AZ: Writing-Review & Editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saleem, S., Jameel, M.H., Alothman, A.A. et al. A band gap engineering for the modification in electrical properties of Fe3O4 by Cu2+ do** for electronic and optoelectronic devices applications. J Sol-Gel Sci Technol 109, 471–482 (2024). https://doi.org/10.1007/s10971-023-06287-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06287-4