Abstract
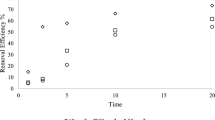
This study investigated the use of ultraviolet (UV) light irradiation for the activation of formic acid (FA) to reduce U(VI) in aqueous solutions. The results demonstrated that under UV radiation, FA exhibited a higher U(VI) reduction efficiency than other organic acids, and up to 70% of U(VI) in aqueous solutions was successfully removed. Electron spin resonance and methanol quenching experiments confirmed that U(VI) reduction in the UV/FA process was due to the electron transfer facilitated by FA (HCOOH) and CO2•−. This catalyst-free UV/FA process holds promise for the reduction of U(VI) in leaking water from uranium tailing ponds.







Similar content being viewed by others
References
Liu B, Peng T, Sun H, Yue H (2017) Release behavior of uranium in uranium mill tailings under environmental conditions. J Environ Radioact 171:160–168
Markich SJ (2002) Uranium speciation and bioavailability in aquatic systems: an overview. Sci World J 2:707–729
O’Loughlin EJ, Kelly SD, Cook RE, Csencsits R, Kemner KM (2003) Reduction of uranium (VI) by mixed iron (II)/iron (III) hydroxide (green rust): formation of UO2 nanoparticles. Environ Sci Technol 37(4):721–727
Augustine S, Gagnaire B, Adam-Guillermin C, Kooijman S (2012) Effects of uranium on the metabolism of zebrafish, Danio rerio. Aquat Toxicol 118–119:9–26
Regulation NPDW (1991) Radionuclides, advanced notice of proposed rulemaking. Federal Regist 56:141–142
Asiabi H, Yamini Y, Shamsayei M (2018) Highly efficient capture and recovery of uranium by reusable layered double hydroxide intercalated with 2-mercaptoethanesulfonate. Chem Eng J 337:609–615
Wang X, Liu Q, Liu J, Chen R, Zhang H, Li R, Li Z, Wang J (2017) 3D self-assembly polyethyleneimine modified graphene oxide hydrogel for the extraction of uranium from aqueous solution. Appl Surf Sci 426:1063–1074
De Decker J, Folens K, De Clercq J, Meledina M, Van Tendeloo G, Du Laing G, Van Der Voort P (2017) Ship-in-a-bottle CMPO in MIL-101(Cr) for selective uranium recovery from aqueous streams through adsorption. J Hazard Mater 335:1–9
Ghasemi Torkabad M, Keshtkar AR, Safdari SJ (2017) Comparison of polyethersulfone and polyamide nanofiltration membranes for uranium removal from aqueous solution. Prog Nucl Energy 94:93–100
Reinoso-Maset E, Ly J (2016) Study of uranium(VI) and radium(II) sorption at trace level on kaolinite using a multisite ion exchange model. J Environ Radioact 157:136–148
Orrego P, Hernández J, Reyes A (2019) Uranium and molybdenum recovery from copper leaching solutions using ion exchange. Hydrometallurgy 184:116–122
Liu X, Liu G, You S (2021) Effective in-situ reduction of Cr(VI) from leather wastewater by advanced reduction process based on CO2•- with visible-light photocatalyst. Chemosphere 263:127898
Li X, Ma J, Liu G, Fang J, Yue S, Guan Y, Chen L, Liu X (2012) Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ Sci Technol 46:7342–7349
Yu H, Nie E, Xu J, Yan S, Cooper WJ, Song W (2013) Degradation of diclofenac by advanced oxidation and reduction processes: kinetic studies, degradation pathways and toxicity assessments. Water Res 47:1909–1918
Jeong J, Song W, Cooper WJ, Jung J, Greaves J (2010) Degradation of tetracycline antibiotics: mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 78:533–540
Flyunt R, Schuchmann MN, Sonntag C (2015) A common carbanion intermediate in the recombination and proton-catalysed disproportionation of the carboxyl radical anion, CO2•-, in aqueous solution. Chem Eur J 7:796–799
DetlefSchröder CA, HelmutSchwarz J, Jeremy NH (1999) On the formation of the carbon dioxide anion radical CO2•- in the gas phase. Int J Mass Spectrom 185:25–35
Rosso JA, Bertolotti SG, Braun AM, Mártire DO, Gonzalez MC (2001) Reactions of carbon dioxide radical anion with substituted benzenes. J Phys Org Chem 14:300–309
**e B, Shan C, Xu Z, Li X, Zhang X, Chen J, Pan B (2017) One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: Reduction to Cr(III) and in situ Cr(III) precipitation. Chem Eng J 308:791–797
Liu X, Zhong J, Fang L, Wang L, Ye M, Shao Y, Li J, Zhang T (2016) Trichloroacetic acid reduction by an advanced reduction process based on carboxyl anion radical. Chem Eng J 303:56–63
Yazdanbakhsh A, Eslami A, Mahdipour F, Ghanbari F, Ghasemi SM, Atamaleki A, Lin KYA (2021) Dye degradation in aqueous solution by dithionite/UV-C advanced reduction process (ARP): kinetic study, dechlorination, degradation pathway and mechanism. J Photochem Photobiol A Chem 407:112995
Mora VC, Rosso JA, Carrillo Le Roux G, Martire DO, Gonzalez MC (2009) Thermally activated peroxydisulfate in the presence of additives: a clean method for the degradation of pollutants. Chemosphere 75:1405–1409
Gu X, Lu S, Fu X, Qiu Z, Sui Q, Guo X (2017) Carbon dioxide radical anion-based UV/S2O82−/HCOOH reductive process for carbon tetrachloride degradation in aqueous solution. Sep Purif Technol 172:211–216
Arslanoğlu H, Altundoğan HS, Tümen F (2021) Photocatalytic reduction of Cr(VI) from aqueous solutions with formic acid in the presence of bauxite: kinetics and mechanism. Trans Indian Inst Met 74:3075–3084
Harbour JR, Hair ML (1979) Spin trap** of the CO2•- radical in aqueous medium. Can J Chem 57:1150–1152
Adams GE (1962) Radiolysis and photolysis of aqueous formic acid carbon monoxide formation. J Am Chem Soc 84:3994
Wang N, Zhu L, Deng K, She Y, Yu Y, Tang H (2010) Visible light photocatalytic reduction of Cr(VI) on TiO2 in situ modified with small molecular weight organic acids. Appl Catal B 95:400–407
Wang X, Rui Z, Ji H (2020) DFT study of formaldehyde oxidation on silver cluster by active oxygen and hydroxyl groups: Mechanism comparison and synergistic effect. Catal Today 347:124–133
Ma R, Yin L, Li L, Zhang S, Wen T, Zhang C, Wang X, Chen Z, Hayat T, Wang X (2018) Comparative Investigation of Fe2O3 and Fe1–xS nanostructures for uranium decontamination. ACS Appl Nano Mater 1:5543–5552
Schwarz HA, Dodson RW (2002) Reduction potentials of CO2•- and the alcohol radicals. J Phys Chem 93:409–414
Draganić Z, Dragani I, Navarro-Gonzáles R, Sehested K, Albarrán-Sanchez M (1991) Radiolysis of aqueous solutions of ammonium bicarbonate over a large dose range. Radiat Phys Chem 38:317–321
Liang C, Su H-W (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562
Golub D, Cohen H, Meyerstein D (1985) Kinetics and mechanism of single electron oxidations of the tervalent uranium ion, U 3+(aq), by free radicals in aqueous solutions. J Chem Soc Dalton Transact 4:641–644
Yu D (1994) Radicals and ions of formic and acetic acids: an Ab initio study of the structures and gas and solution phase thermochemistry. Org Biomol Chem 1:2207–2215
Lucks C, Rossberg A, Tsushima S, Foerstendorf H, Fahmy K, Bernhard G (2013) Formic acid interaction with the uranyl(VI) ion: structural and photochemical characterization. Dalton Trans 42:13584–13589
Acknowledgements
This work was supported by and the Key projects of key R & D plan of Jiangxi Province (20212BBG71011), the National Natural Science Foundation of China (21966004, 22006011, 52064001) and the Natural Science Foundation of Jiangxi, China (20224BAB203028, 20202ACBL213006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, D., Guo, Y., Wang, J. et al. Research on the uranium (VI) reduction by free radicals using UV/formic acid process. J Radioanal Nucl Chem 332, 4441–4447 (2023). https://doi.org/10.1007/s10967-023-09138-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09138-2