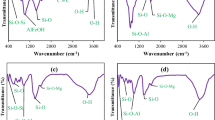
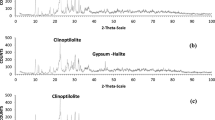
Abstract
This study investigated the feasibility of using montmorillonite and kaolinite to remediate uranium-contaminated groundwater. The results showed that the two minerals achieved U(VI) removal rates of 78.68% and 69.89%, respectively. The maximum saturation adsorption amounts were 3.78 × 10–5 mol g−1 and 3.85 × 10–5 mol g−1, respectively. The Langmuir model and pseudo-second-order kinetics can describe the adsorption process of kaolinite on U(VI). The adsorption process of montmorillonite on U(VI) was well-fitted by Freundlich, and pseudo-second-order models. Thermodynamic parameters indicated that the two minerals' adsorption of U(VI) was a heat-absorbing reaction. The results of SEM–EDS, FT-IR revealed that the adsorption of U(VI) by the two minerals was mostly an ion-exchange reaction, and various functional groups were involved in the adsorption process. XPS results showed valence changes accompanied by kaolinite adsorption of U(VI). Montmorillonite and kaolinite have some differences in the adsorption process of U(VI).

















Similar content being viewed by others
References
Minas F, Chandravanshi BS, Leta S (2017) Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem Int 3(4):291–305
Kharecha PA, Hansen JE (2013) Prevented mortality and greenhouse gas emissions from historical and projected nuclear power. Environ Sci Technol 47:4889–4895
Xun Y, Xuegang L (2015) Radionuclides distribution, properties, and microbial diversity of soils in uranium mill tailings from southeastern China. J Environ Radioact 139:85–90
Sun Z, Chen D, Chen B, Kong L, Su M (2018) Enhanced uranium(VI) adsorption by chitosan 394 modified phosphate rock. Colloid Surf A 547:141–147
Chen S, Wei X, Liu J, Sun Z, Chen G, Yang M, Liu Y, Wang D, Ma C, Kong D (2022) Weak acid leaching of uranium ore from a high carbonate uranium deposit. J Radioanal Nucl Chem Int J Deal Asp Appl Nucl Chem 6:331
Orozco I, Romero M, Lara R, Bazan V (2018) Precipitation of uranium from alkaline liqueurs. Matéria 23:3336
Rong L, Zeming S, Yun H, Kailiang Z, Lvhang Y (2019) Uranium sorption onto mullite: characteristics of isotherms, kinetics and thermodynamics. J Earth Syst Sci 128(7):176–179
Sar SK, Diwan V, Biswas S, Singh S, Sahu M, **dal MK, Arora A (2018) Study of uranium level in groundwater of Balod district of Chhattisgarh state, India and assessment of health risk. Hum Ecol Risk Assess 24:691–698
Yang H, Luo X, Ding H, Zhang X (2018) Adsorption of U(VI) by elodea nuttallii: equilibrium, kinetic and mechanism analysis. J Radioanal Nucl Chem 319(4):227
Hamutoko JT, Mapani BS, Ellmies R, Bittner A, Kuells C (2014) A fngerprinting method for the identifcation of uranium sources in alluvial aquifers: an example from the Khan and Swakop Rivers, Namibia. Phys Chem Earth 72–75:34–42
Nada FT, Laith AN, Enas MY (2014) Uranium concentration and its associated health hazards in drinking water of Nineveh Province (Iraq). World Appl Sci J 31(11):1938–1944
Borch T, Roche N, Johnson TE (2012) Determination of contaminant levels and remediation efficacy in groundwater at a former in situ recovery uranium mine. J Environ Monit JEM 14(7):1814–1823
Obiri-Nyarko F, Grajales-Mesa SJ, Malina G (2014) An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 111:243–259
LIZhang JY (2012) Remediation technology for the uranium contaminated environment: a review. Proc Environ Sci 13:1609–1615
MaZhang JY (2006) Simultaneous sorption of phosphate and phenanthrene to inorgano-organo-bentonite from water. J Hazard Mater 136(3):982–988
Ma L (2014) Als-pill ared montmorillonite modifed by cationic and zwitterionic surfactants: a comparative study. Appl Clay Sci 101:327–334
Diwan V, Sar SK, Biswas S, Lalwani R (2020) Adsorptive extraction of uranium(VI) from aqueous phase by dolomite. Groundw Sustain Dev 11:100424
Kornilovych B, Kovalchuk L, Tobilko V, Ubaldini S (2020) Uranium removal from groundwater and waste water using clay-supported nanoscale zero-valent iron. Metals 10:111–116
**ong H, JieminZhang T, WenzhongHuang C, YulingHu B (2021) Unexpected ultrafast elimination of uranium and europium from aqueous solutions with magnetic bio-CaCO3. J Mol Liquids 322(1):114986
Khan S, Anjum R, Bilal M (2021) Revealing chemical speciation behaviors in aqueous solutions for U(VI) and europium (III) adsorption on zeolite. Environ Technol Innov 22:101503
Gladysz-Plaska A (2019) Adsorption properties of sepiolite in relation to Uranium and Lanthanide lons. Imerals 5:20–31
Bergaya F, Lagaly G (2006) Chapter 1 general introduction: clays, clay minerals, and clay science. Dev Clay 1(05):1–18
Zhu R, Chen Q, Zhou Q, ** Y, Zhu J, He H (2016) Adsorbents based on montmorillonite for contaminant removal from water: a review. Appl Clay Sci 123:239–258
Kralik M (2014) Adsorption, chemisorption, and catalysis. Chemicke Zvesti 68(12):1625–1638
Zhang N, Lin LS, Gang DC (2008) Adsorptive selenite removal from water using iron-coated GAC adsorbents. Water Res 42:3809–3816
Liu B, Sun H, Peng T, Duan T (2019) Transport and transformation of uranium and heavy metals from uranium tailings under simulated rain at different pH. Environ Chem Lett 18:10
Wang X (2016) Study on the adsorption behavior of Th(IV) and U(VI) on two different illites[D]. Lanzhou University
Grim RE (1953) Clay mineralogy. McGraw-Hill, New York
Majd MM, Kordzadeh-Kermani V, Ghalandari V, Askari A, Sillanp M (2022) Adsorption isotherm models: a comprehensive and systematic review (20102020). Sci Total Environ 812:151334–152338
Kapnisti M, Noli F, Misaelides P, Vourlias G, Karfaridis D, Hatzidimitriou A (2018) Enhanced sorption capacities for lead and uranium using titanium phosphates; sorption, kinetics, equilibrium studies and mechanism implication. Chem Eng J 342:184–195
Yang C, Zhong Y, Li L, Ren X, Sun Y, Niu D, Liu Y, Yin M, Zhang D (2018) Lead and uranium sorption characteristics on hydrothermal synthesized delta manganese dioxide. J Radioanal Nucl Chem Int J Deal Aspect Appl Nucl Chem 317(3):1399–1408
Wang J, Guo X (2020) Adsorption kinetic models: physical meanings, applications, and solving methods. J Hazard Mater 15(390):122156
Noreen S, Kausar A, Zaman Q, Bhatti H, Nawaz I (2016) Efficient remediation of zr(IV) using citrus peel waste biomass: kinetic, equilibrium and thermodynamic studies. Ecol Eng J Ecotechnol 95:216–228
Ali SI, Lalji SM, Awan Z, Hashmi S, Khan G, Asad M (2022) Comprehensive performance analysis of kinetic models used to estimate asphaltene adsorption kinetics on nanoparticles. Chem Papers 77:1–15
Lebedeva OV, Sipkina EI et al (2016) Adsorption of Platinum(IV) by a composite based on silicon dioxide and copolymer of 4-Vinylpyridine and 2-Hydroxyethylmethacrylate. Protect Metals Phys Chem Surf 53(2017):80–84
Cheira MF, Mira HI, Sakr AK, Mohamed SA (2019) Adsorption of U(VI)from acid solution on a low-cost sorbent: equilibrium, kinetic, and thermodynamic assessments. Nucl Technol Engl 30(10):18
Meng F, Yuan G, Larson SL, Ballard JH, Han FX (2019) Kinetics and thermodynamics of uranium (VI) adsorption onto humic acid derived from leonardite. Int J Environ Res Public Health 16(9):1552
Tseng KNT, Kampf JW, Szymczak NK (2015) On the mechanism of n,n,n-amide ruthenium(II) hydride mediated acceptorless alcohol dehydrogenation: inner-sphere β-h elimination vs. outer-sphere bifunctional metal-ligand cooperativity. Acs Catal, 150810145618005
Gado MA, Atia BM, Hagag MS (2020) Kinetics and thermodynamics of uranium adsorption using impregnated magnetic graphene oxide. Iran J Chem Chem Eng Int Engl Edn 39:225
Elhefnawy OA, Elabd AA (2017) Optimization of uranyl ions removal from aqueous solution by natural and modified kaolinites. Radiochim Acta 105:609
Li JH, Liu WG, Zhang ZM, Li HN, Jiang QF, Wang YC, Tang RJ, Xu B, Guo RH, Su XB, Hua R (2022) Adsorption of uranium on amino functionalized acrylonitrile anion exchange resin. J Radioanal Nucl Chem 331(12):5771–5779
Peng TQ, Wang YF, Xu YF, Liu ZC (2023) Synthesis, characterization and uranium (VI) adsorption mechanism of novel adsorption material poly(tetraethylenepentamine-trimesoyl chloride). J Radioanal Nucl Chem 332(2):409–422
Vahur S et al (2021) Quantitative mineralogical analysis of clay-containing materials using atr-ft-ir spectroscopy with pls method. Anal Bioanal Chem 26:6535
Zhu M, Liu L, Feng J, Dong H, Zhang C, Ma F, Wang Q (2021) Efficient uranium adsorption by amidoximized porous polyacrylonitrile with hierarchical pore structure prepared by freeze-extraction. J Mol Liquids 328:115304
**g C, Li Q, Tang Z, Xu J, Li Y (2019) Removal of soluble uranium by Illite supported nanoscale zero-valent iron: electron transfer processes and incorporation mechanisms. J Radioanal Nucl Chem 323:2016
Peng TQ, Wang YF, Xu YF, Liu ZC (2023) Synthesis, characterization and uranium (VI) adsorption mechanism of novel adsorption material poly(tetraethylenepentamine-trimesoyl chloride). J Radioanal Nucl Chem 332:1–14
Zhou W, Wang J, He J, Yang X, Liu C (2019) Adsorption of U(VI) on montmorillonite in the presence of ethylenediaminetetraacetic acid. Colloids Surf A 583:123929
Senol ZM, Keskin ZS, Ozer A, Simsek S (2022) Application of kaolinite-based composite as an adsorbent for removal of uranyl ions from aqueous solution: kinetics and equilibrium study. J Radioanal Nucl Chem Int J Deal All Aspect Appl Nucl Chem 1:331
Hagag MS, Esmaeel SMA, Salem F, Ali AH, Zaki SA (2022) Thermal activation of kaolinite titanium hydroxide composite for uranium adsorption from aqueous solutions. Int J Environ Sci Technol 3:1–12
Geng R, Yuan L, Shi L, Qiang S, Li Y, Liang J, Li P, Zheng G, Fan Q (2022) New insights into the sorption of U(VI) on kaolinite and illite in the presence of aspergillus niger. Chemosphere 288:132497
Wu J, Zheng Z, Zhu K, **ang C, Wang J, Liu J (2022) Adsorption performance and mechanism of g-c3n4/uio-66 composite for u(vi) from aqueous solution. J Radioanal Nucl Chem 331(1):469–481
Acknowledgements
The authors are extremely thankful to the anonymous reviewers that work in this paper.
Funding
National Natural Science Foundation of China (No. 42272301). Open Fund Project of State Key Laboratory of Nuclear Resources and Environment, East China University of Technology (No.2020NRE18). Foundation for Educational Commission of Jiangxi Province of China (No. GJJ200720). Open Fund of National Defense Key Discipline Laboratory of Radioactive Geology and Exploration Technology (No. 2020RGET08).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Gao, B., Ma, W. et al. Different behavior of uranium(VI) on two clay minerals: montmorillonite and kaolinite. J Radioanal Nucl Chem 332, 4029–4046 (2023). https://doi.org/10.1007/s10967-023-09119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09119-5