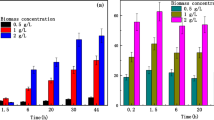
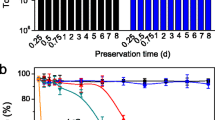
Abstract
Radionuclide recycling by biosorption combined with the ashing process is a promising nuclide recovery method. To investigate the transformation process of radionuclide occurrence state in bio-recycling, uranium and strontium recycling by S.cerevisiae were studied. S. cerevisiae exhibits good performance in the enrichment of uranium and strontium with as high as almost 90% biosorption efficiency. The results demonstrate that adsorbed uranium and strontium precipitates can be transformed into authenite and strontium sulfate on cell surface. The final state of uranium is mainly in form of UP2O7, while the final state of Sr(II) is mainly in form of SrSO4 after ashing.
Graphic abstract







Similar content being viewed by others
References
Agency ONE (2007) Nuclear Development Risks and Benefits of Nuclear Energy: Complete Edition - ISBN 9264035516. Sourceoecd Nuclear Energy, volume 2007: p. i-88(89)
Kochkin B et al (2021) Problems and perspectives of borehole disposal of radioactive waste. Prog Nucl Energy 139:103867
Ferenbaugh JK et al (2002) Radionuclides in soil and water near a low-level disposal site and potential ecological and human health impacts. Environ Monitor Assess 74:243
Banala UK, Das NPI, Toleti SR (2021) Microbial interactions with uranium: Towards an effective bioremediation approach. 101254Environ Technol Innov 21:101254
Liu M et al (2010) Biosorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions. Bioresour Technol 101(22):8573–8580
Hu W et al (2017) Synergistic interface behavior of strontium adsorption using mixed microorganisms. Environ Sci Pollut Res Int 25:22368
Zhang J et al (2020) Uranium biosorption mechanism model of protonated Saccharomyces cerevisiae. J Hazard Mater 385:121588
Liu M et al (2016) Programmed gradient descent biosorption of strontium ions by Saccaromyces cerevisiae and ashing analysis: a decrement solution for nuclide and heavy metal disposal. J Hazard Mater 314:295–303
Chen L et al (2021) Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: a review. J Hazard Mater 413:125319
Farhan SN, Khadom AA (2015) Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int J Ind Chem 6(2):119–130
Altimari P, Caprio FD, Pagnanelli F (2017) Biosorption of Copper by Saccharomyces cerevisiae: From Biomass Characterization to Process Development.
Fadel M et al (2017) Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. Hbrc J 13(1):106–113
Qiu L et al (2017) Biosorption of the strontium ion by irradiated Saccharomyces cerevisiae under culture conditions. J Environ Radioact 172:52–62
Zheng XY et al (2017) Biosorption and biomineralization of uranium(VI) by Saccharomyces cerevisiae-crystal formation of chernikovite. Chemosphere 175:161–169
Liu M et al (2014) Biosorption of strontium from simulated nuclear wastewater by Scenedesmus spinosus under culture conditions: adsorption and bioaccumulation processes and models. Int J Environ Res Public Health 11(6):6099–6118
Shen Y et al (2018) The biomineralization process of uranium(VI) by Saccharomyces cerevisiae—transformation from amorphous U(VI) to crystalline chernikovite. Appl Microbiol Biotechnol 102:4217
Hu W et al (2017) Synergistic interface behavior of strontium adsorption using mixed microorganisms. Environmental Science & Pollution Research
Pathirana C et al (2022) Biosorption of heavy metals: transferability between batch and column studies. Chemosphere 294:133659
** Y, Macaskie LE (2010) Removal of the tetravalent actinide thorium from solution by a biocatalytic system. J Chem Technol Biotechnol Biotechnol 64(1):87–95
Şimşek S, Yılmaz E, Boztuğ A (2013) Amine-modified maleic anhydride containing terpolymers for the adsorption of uranyl ion in aqueous solutions. J Radioanal Nucl Chem 298(2):923–930
Şenol ZM et al (2021) Synthesis and characterization of chitosan–vermiculite composite beads for removal of uranyl ions: isotherm, kinetics and thermodynamics studies. J Radioanal Nucl Chem 327(1):159–173
Monnin C (1999) A thermodynamic model for the solubility of barite and celestite in electrolyte solutions and seawater to 200°C and to 1 kbar. Chem Geol 153(1):187–209
Monnin C, Galinier C (1988) The solubility of celestite and barite in electrolyte solutions and natural waters at 25°C: A thermodynamic study. Chem Geol 71(4):283–296
Merroun ML et al (2005) Complexation of uranium by cells and S-layer sheets of Bacillus sphaericus JG-A12. Appl Environ Microbiol 71(9):5532
Krueger S et al (1993) Characterization of the Binding of Gallium, Platinum, and Uranium to Pseudomonas fluorescens by Small-Angle X-Ray Scattering and Transmission Electron Microscopy, vol 59. Applied & Environmental Microbiology, pp 4056–4064. 12
Wang T et al (2017) Different biosorption mechanisms of Uranium(VI) by live and heat-killed Saccharomyces cerevisiae under environmentally relevant conditions. J Environ Radioact 167:92–99
Shao XZ (2010) Biosorption of strontium ions by magnetically modified yeast cells. Sep Sci Technol 45(10):1499–1504
Guo Y et al (2016) The biosorption of Sr(II) on Bacillus subtilis: a combined batch and modeling study. J Mol Liq 220:762–767
Wang Q et al (2007) Alginate/polyethylene glycol blend fibers and their properties for drug controlled release. J Biomedical Mater Res Part A 82(1):122–128
Gniadecka M et al (2004) Melanoma diagnosis by Raman spectroscopy and neural networks: structure alterations in proteins and lipids in intact cancer tissue. J Invest Dermatology 122(2):443–449
Huang CY, Balakrishnan G, Spiro TG (2006) Protein secondary structure from deep-UV resonance Raman spectroscopy. J Raman Spectrosc 37(1–3):277–282
Barth A (2000) The infrared absorption of amino acid side chains. Prog Biophys Mol Biol 74(3):141–173
Schwanninger M et al (2004) Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spectrosc 36(1):23–40
Ramos ML et al (2017) Oxocomplexes of U (vi) with 8-hydroxyquinoline-5-sulfonate in solution: structural studies and photophysical behaviour. Dalton Trans 46:9358–9368
Fan X, Xu N-J, Shi J-G (2003) Bromophenols from the Red Alga Rhodomela confervoides. J Nat Prod 66(3):455–458
Palaniappan PR, Pramod K (2010) FTIR study of the effect of nTiO2 on the biochemical constituents of gill tissues of Zebrafish (Danio rerio). Food Chem Toxicol 48(8):2337–2343
Takagi H (2021) Molecular mechanisms and highly-functional development for stress tolerance of the yeast Saccharomyces cerevisiae. Biosci Biotechnol Biochem 85:1017–1035
Zhang J, Kirkham M (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35(5):785–791
Demiral T, Türkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53(3):247–257
Wilmsen PK, Spada DS, Salvador M (2005) Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem 53(12):4757–4761
Acknowledgements
This study was supported by the National Natural Science Foundation of China (42007281, 51974261), the Key Project of National Natural Science Foundation of China (41831285), and the National Key Research and Development Program (2018YFC1903304).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., Dong, F., Dai, Q. et al. Transformation of radionuclide occurrence state in uranium and strontium recycling by Saccharomyces cerevisiae. J Radioanal Nucl Chem 331, 2621–2629 (2022). https://doi.org/10.1007/s10967-022-08308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08308-y