Abstract
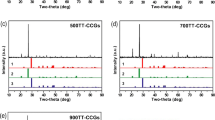
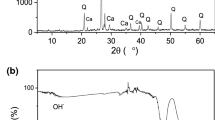
The preparation of C30 concrete for a cavernous waste repository under construction in China and its Cs(I) adsorption performance were investigated. The mix ratio of C30 concrete was cement:sand:gravel:water = 1:1.87:3.05:0.55. Three characterization analyses showed that C30 concrete has a good crystal structure, a large specific surface area and the presence of many –OH, HCO3−, CO32−, SiO42− groups and other groups. Adsorption experiments showed that the adsorption equilibrium was reached within 4 h, and pH had little effect on adsorption; several ions (K+, Ca2+, Mg2+, Cl−, NO3−, and SO42−) inhibited adsorption. The adsorption was more in line with the pseudo-second-order kinetic model and the Langmuir isothermal model. Thermodynamic analysis indicates that adsorption is endothermic and stochastic.









Similar content being viewed by others
References
Lei X (2015) The removal of uranium and cobalt from wastewater through adsorption by mesoporous calcium silicate material. Tsinghua University
Dm A, Ms B, Sp B (2020) Design, structure, microstructure and gamma radiation shielding properties of refractory concrete materials containing Ba- and Sr-doped cements - ScienceDirect. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2020.124095
Alam MS, Gorman-Lewis D, Chen N, Safari S, Baek K, Konhauser KO, Alessi DS (2018) Mechanisms of the removal of U(VI) from aqueous solution using biochar: a combined spectroscopic and modeling approach. Environ Sci Technol. https://doi.org/10.1021/acs.est.8b01715
Mahmoud MA (2021) Sorption of U(VI) ions from aqueous solution by eggplant leaves: isotherm, kinetics and thermodynamics studies. Prog Nucl Energy. https://doi.org/10.1016/j.pnucene.2021.103829
Li P, Zhun B, Wang XG, Liao PP, Wang GH, Wang LZ, Guo YD, Zhang WM (2017) Highly efficient interception and precipitation of uranium(VI) from aqueous solution by iron-electrocoagulation combined with cooperative chelation by organic ligands. Environ Sci Technol. https://doi.org/10.1021/acs.est.7b05288
Mo G, Hu Q, Wang G, **e S, Nong H, Zhang X, Zeng T (2021) Fe3O4-modifed sewage sludge biochar for U(VI) removal from aqueous solution: performance and mechanism. Radioanal Nucl Chem. https://doi.org/10.1007/s10967-021-07782-0
Yu J, Wang XH, ** YR (2011) Adsorption partition coefficient of 239Pu on mudstone and sandstone. Chin J Nucl Radiochem 33(3):173–178
Wang J, Chen W, Su R, Guo Y, ** Y (2006) Geological disposal of high-level radioactive waste and its key scientific issues. Chin J Rock Mech Eng 25(4):801–812
Hong S-W, Kim MS, Jung KII, Park JB (2017) Determination of radionuclide concentration limit for low and intermediate-level radioactive waste disposal facility II: application of optimization methodology for underground silo type disposal facility. J Nucl Fuel Cycle Waste Technol. https://doi.org/10.7733/jnfcwt.2017.15.3.265
Zheng W, Cheng X (2014) Research on related problems of low and intermediate level radioactive waste disposal in China. Chin J Energy Constr. https://doi.org/10.16516/j.gedi.ieen2095-8676.2014.01.01
Jiang LG, Tian JB, Liu YF (1989) Adsorption behavior of concrete and clay on zirconium. Chin J Nucl Radiochem 11(3):189–189
Kim WS, Han S, Ahn J (2019) Investigation of 3H, 99Tc, and 90Sr transport in fractured rock and the effects of fracture-filling/coating material at LILW disposal facility. Environ Geochem Health. https://doi.org/10.1007/s10653-018-0123-y
Jelić I, Šljivić-Ivanović M, Dimović S (2019) Radionuclide immobilization by sorption onto waste concrete and bricks-experimental design methodology. Water Air Soil Pollut. https://doi.org/10.1007/s11270-019-4298-3
Zhang PC, Li J, Ni SJ (2008) Study on the adsorption of 238U, 90Sr and 137Cs on the surrounding rock of a very low level radioactive waste disposal site. Chin J Geophys Geochem Comput Technol 30(4):5
JGJ 55-2011 Specification for mix proportion design of ordinary concrete (in China)
Fan Y (2017) Construction materias. Bei**g Institute of Technology Press (in China)
dos Reis GS, Thue PS, Cazacliu BG, Lima EC, Sampaio CH, Quattrone M, Ovsyannikova E, Kruse A, Dotto GL (2020) Effect of concrete carbonation on phosphate removal through adsorption process and its potential application as fertilizer. J Pre-proof. https://doi.org/10.1016/j.jclepro.2020.120416
GBT50081-2019 Standard for test methods of concrete physical ancmechanical properties (in China)
Xu D, Zuo R, Han K, Ding F, **a S, Zhao X, Shia R, Wang J (2020) Sorption of Sr in granite under typical colloidal action. Contam Hydrol. https://doi.org/10.1016/j.jconhyd.2020.103659
Cheira MF, Atia BM, Kouraim MN (2017) Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: kinetic, equilibrium and thermodynamic studies. Radiat Res Appl Sci. https://doi.org/10.1016/j.jrras.2017.07.005
Kang S, Lee J, Park S-M, Alessi DS, Baek K (2020) Adsorption characteristics of cesium onto calcium-silicate-hydrate in concrete powder and block. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127494
Bertuoli PT, Piazza D, Scienza LC, Zattera AJ (2014) Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl Clay Sci. https://doi.org/10.1016/j.clay.2013.11.020
JunShuai D (2020) The influence of sea sand and sea water on the performance of cement mortar. Wuhan Textile University
Moreira MA, Ciuffi KJ, Rives V, Vicente MA, Trujillano R, Gil A, Korili SA (2017) Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl Clay Sci. https://doi.org/10.1016/j.clay.2016.10.022
Yan KY (2019) Research on influences of nanomaterials on calcium silicate hydrates and cement-based materials. Harbin Institute of Technology
Lin S (2019) Adsorption of Eu(III), Th(IV) and Se(IV) on Beishan granite. Harbin Institute of Technology
Wamba AGN, Lima EC, Ndi SK, Thue PS, Kayem JG (2017) Synthesis of grafted natural pozzolan with 3-aminopropyltriethoxysilane: preparation, characterization, and application for removal of Brilliant Green 1 and Reactive Black 5 from aqueous solutions. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-9825-4
Volchek K, Miah MY, Kuang W, DeMaleki Z, Tezel FH (2011) Adsorption of cesium on cement mortar from aqueous solutions. Hazard Mater 194:331–337
Miah MY, Volchek K, Kuang W, Tezeld FH (2010) Kinetic and equilibrium studies of cesium adsorption on ceiling tiles from aqueous solutions. Hazard Mater. https://doi.org/10.1016/j.jhazmat.2011.07.111
Chen X, **e S, Wang G, Liu H, Guo Y, Yang S, Wu S, Liu X (2021) The performance and mechanism of U(VI) removal from aqueous solutions by a metal–organic framework (DUT-69). J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-021-07645-8
Roshdy E (2021) Removal of uranium, cadmium and iron ions from phosphoric acid solution using amberjet 1200 H resin: an experimental, isotherm and kinetic study. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-021-07792-y
**ao F, Cheng Y, Zhou P, Chen S, Wang X (2021) Fabrication of novel carboxyl and amidoxime groups modified luffa fiber for highly efficient removal of uranium(VI) from uranium mine water. Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105681
Soliman AM, Madbouly HA, El Sheikh ES, Khalil M, Massad A (2021) Selective removal and immobilization of cesium from aqueous solution using sludge functionalized with potassium copper hexacyanoferrate: a low-cost adsorbent. J Radioanal Nucl Chem. https://doi.org/10.1007/510967-021-07964-w
Acknowledgements
Financial supports from National Natural Science Foundation of China (No. 41630646) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, G., Wang, Y., Yang, X. et al. Preparation of C30 concrete and its adsorption performance for Cs(I). J Radioanal Nucl Chem 331, 2135–2145 (2022). https://doi.org/10.1007/s10967-022-08273-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08273-6